Neuquenaphis ramirezi Nieto Nafría & Ortego, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4590.5.2 |
|
publication LSID |
lsid:zoobank.org:pub:685DF8B3-F249-40CB-AA50-EF931C287FB8 |
|
persistent identifier |
https://treatment.plazi.org/id/AC4687AA-9B7F-C523-17DC-A15DFD96F87C |
|
treatment provided by |
Plazi |
|
scientific name |
Neuquenaphis ramirezi Nieto Nafría & Ortego |
| status |
sp. nov. |
Neuquenaphis ramirezi Nieto Nafría & Ortego , sp. n.
2003. Blackman, R.L., Brown, P.A., Ramírez, C.C., Niemeyer, H.M.: Neuquenaphis “sp. B”.
2018. Blackman, R.L. and Eastop V.F.: Neuquenaphis sp. B of Blackman et al. (2003).
Types. Holotype: apterous viviparous female (number 27 of measurement series), CHILE, Aysén region, General Carrera province: Jeinimeni lake [approx. 46°50’S 72°01’W, 850 m], 9 December 2001, on Nothofagus pumilio, Brown and Villagra et al. leg. Natural History Museum London collection GoogleMaps . Paratypes: 57 apterous viviparous females [apt.] . CHILE, La Araucanía region, Cautín province, Conguillio National Park [approx. 38°50’S 71°37’W, 520 m], 9 December 2000, on N. pumulio , 1 apt., P. A. Brown & C. Ramírez leg., Karyotype number RLB4575 GoogleMaps . CHILE, Aysén region, same data than holotype, 8 apt. GoogleMaps ; CHILE, Aysén region, same locality and collectors than the holotype, 25 November 2001, on Nothofagus antarctica and on N. pumilio 4 plus1 apt., respectively ; 26 November 2001, on N. pumilio , 1 apt. ; 8 December 2001, on N. pumilio , 1 apt. ; CHILE, Aysén region, General Carrera province: Verde lake [approx. 46°51’S 72°04’W, 860 m], 2 December 2001, 3 apt., 6 December 2001, 6 apt., Brown and Villagra et al. leg GoogleMaps .. CHILE, Magallanes region, Magallanes province, Las Vacas [52°22’S 71°25’W, 290m], 4 February 2016, on N. pumilio , 6 apt., Nieto Nafría, Mier Durante and Ortego leg. GoogleMaps ; CHILE, Magallanes region, Última Esperanza province, Guardería Grey [51°07’S 73°07’W, 60m], 5 February 2016, on N. pumilio , 4 apt., same collectors GoogleMaps ; CHILE, Magallanes region, Pehoé island [51°05’S 72°59’W, 40m], 5 February 2016, on N. pumilio , 22 apt., same collectors. Natural History Museum London and University of León collections. GoogleMaps
Etymology. The specific name of the new species, ramirezi , is devoted to Claudio C. Ramírez Rivera. (associate professor of the University of Talca, Chile), who is coauthor of several papers on Chilean Neuquenaphis species ( Quiroz et al., 1999; Blackman et al., 2003; Gaete-Eastman et al., 2004; Ramírez et al., 2008) and who also collected specimens.
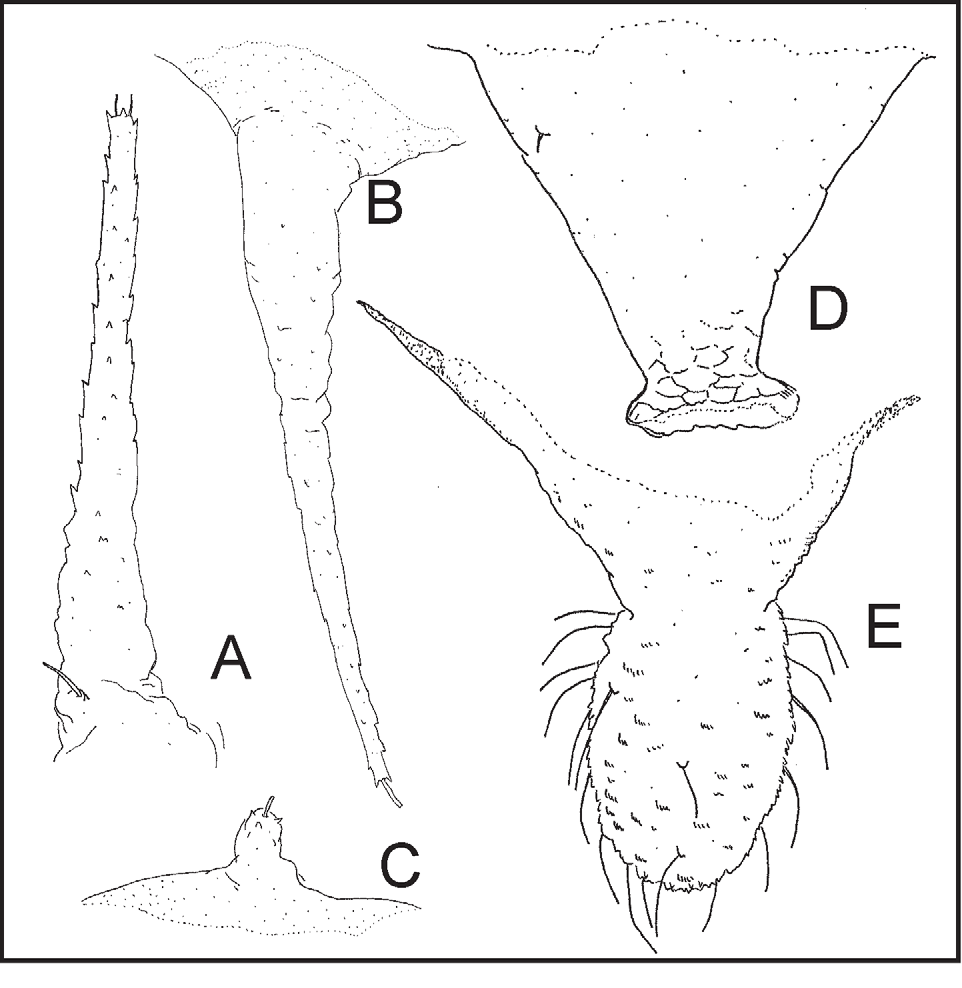
Description. Apterous viviparous females ( Figures 5 View FIGURE 5 , 6 View FIGURE 6 ). From 58 specimens, 56 of which were measured. When alive dark green or greenish brown without spots, or with spots more-or-less dark. Body 1.75–2.25 mm. Other measurements are in Table 2 View TABLE 2 . Mounted specimens variably pigmented. In few pigmented specimens (perhaps the pallor of some specimens is an artefact of the preservation process) extensive areas of the body dorsum very pale, with (1) finger-shaped processes, anal plate and cauda pale cream, (2) cephalic dorsum and most part of prothoracic dorsum, marginal zones of meso- and metathorax, basal sclerites of finger-shaped processes and spiracular sclerites pale cream to cream, and (3) siphunculi and intersegmental muscular sclerites dark cream. In darker specimens on a pale background (1) head, dorsally and ventrally, basal sclerites of the marginal and submarginal abdominal finger-shaped processes, transverse bands on abdominal segments 7 and 8, spiracular sclerites, anal plate and cauda pale brown to brown, (2) dorsum of pro- and mesothorax, basal sclerites of metathoracic finger-shaped processes (sometimes coalescing with each other), basal sclerites of abdominal segments 1–6 with finger-shaped processes (also sometimes coalescing with each other), intersegmental sclerites and siphunculi brown to dark brown, usually the area in immediate contact with processes is less pigmented than the rest or is even pale. In all specimens, antennal segments I–IV, most of antennal segment V, rostrum and legs pale cream to cream; antennal segment VI and apex of segment V dark cream to brown or dark brown. No wax pores observed. All dorsal processes with proximal rings of wrinkles and with scarce and feeble spines, those of the apical ring shorter than the distal setae, which are short and widened and irregularly blunt at apex. Head with two pairs of finger-shaped processes, anterior one with two apical setae on each process. Sometimes the seta close the base of anterior finger-shaped process is sited on the basal part of the process. Frons nearly flat; epicraneal line usually inconspicuous on few pigmented specimens. Ventral head more or less wrinkled and with two lateral setae, delicate and pointed and with the typical medial bulge, oval, rugous and with two pairs of setae, thick and also pointed. Antennae usually longer than body. Antennal segment I almost smooth, with 6–15 thick and pointed setae. Antennal flagellum progressively imbricate from almost smooth proximal half of segment III. Inner margin of antennal segment III with 3–9 setae, pointed and thinner than those of segment I. Base of antennal segment VI with 1–3 setae, fine and pointed. Primary and satellite sensoria ciliate; several satellite sensoria distal to primary sensorium. Rostrum reaching beyond middle coxae. Ultimate rostral segment relatively long and with 2–8 accessory setae, long and delicate. Prothorax with four spinal finger-shaped processes in two rows, a postero-lateral bulge, broad, rough and carrying a wart-shaped process or tubercle or elevation on its anterior half, and usually wart-shaped processes pleural and submarginal, also variable in size. Mesothorax and metathorax with three pairs of thin finger-shaped processes (spinal, pleural and marginal) on each. Legs slender. Femora with scarce setae, short, delicate and pointed. Tibiae smooth or nearly smooth, with setae more abundant, longer and robust than those in femora and also pointed. First tarsal segments with 2 dorsal (sometimes lost or lacking) and 7 ventral setae, long and delicate. Second tarsal segment imbricated. Empodial setae spatulate. Abdominal segments 1 to 6 always with three pairs of thin finger-shaped processes (spinal, pleural and marginal) on each, and with finger-shaped or wart-shaped process or elevations on all or several of them. Siphunculus almost smooth, with 1 (sometimes incomplete) to 3 rows of cells at the apex and broad flange. Abdominal segment 7 with two pairs of finger-shaped processes; abdominal segment 8 with a single pair of finger-shaped processes and 2 in all marginal setae, delicate and pointed. Knob of cauda elongated, with (17)20–26(33) setae, long and delicate.
Alate viviparous females. Still unknown.
First instar nymph (or old embryo). From 2 specimens. Antennae with 5 segments, II and VI (base) with 2 setae each. Ultimate rostral segment without accessory (or secondary) setae. Anterior cephalic processes fingershaped and bifurcate in two short branches. Prothorax with four spinal finger-shaped processes and two marginal wart-shaped processes. Mesothorax and metathorax with spinal and pleural finger-shaped processes and marginal wart-shaped processes. Abdominal segment 1 to 6 with 3 pairs (spinal, pleural and marginal) of finger-shaped processes, segment 7 with two pairs and segment 8 with one pair. All processes scarcely spinuled, with a crown of spines, relatively long (approximately 4–5 times width at middle), blunt or enlarged at apex, smooth and with minuscule setae. Anal segment with 2 setae.
Karyotype. From Blackman et al. (2003), females number 2n = 16.
Nucleotide sequences. Neighbor-joining dendrograms generated from the COI locus placed this species as most similar to N. similis and N. edwardsi ( Figure 7 View FIGURE 7 ). Of the latter, N. ramirezi sp. n. was genetically closer to N. similis (10.2% for N. similis vs. 12.3% for N. edwardsi ). N. ramirezi sp. n. is genetically well differentiated from the remaining Neuquenaphis species sampled, with distances ranging from 10.2–21.5% ( Table 3 View TABLE 3 ).
Biology. Neuquenaphis ramirezi sp. n. has been collected on Nothofagus pumilio (Poepp. & Endl.) Krasser. and less frequently on N. antarctica (G.Forst.) Oerst. These trees are deciduous and sexuals of other Neuquenaphis species have been described, so it is probable that the life cycle of this species is holocyclic, although oviparous females and males have not yet been collected.
Distribution. Neuquenaphis ramirezi sp. n. has been recorded from six localities of Southern Chile; the northernmost (near the 38° S parallel) and the southernmost (near the 52° S parallel) localities delimit a wide area of distribution for the new species, which seems to be more frequent in the southern parts. Most of these localities are close to lakes or to the sea.
Taxonomic discussion, diagnostic characteristics. Neuquenaphis ramirezi sp. n. must be included in the nominotypical subgenus. Dorsal-abdominal sclerotisation and pigmentation of apterous viviparous females of N. ramirezi sp. n. are similar to that of apterae of N. sensoriata , and apterae of early generations of N. edwardsi , but in the latter two species submarginal processes are unusual, and if they are present then they are short wart-shaped processes or cuticular elevations. In addition, apterous viviparae of N. sensoriata and N. edwardsi have antennae conspicuously shorter than body (at least 0.94 times body length in N. ramirezi ) and relatively short antennal segment VI processus terminalis. Apterous viviparae of N. sensoriata , in which the diploid chromosome number is the same as N. ramirezi (16), have wider dorsal pigmentation and robust finger-shaped processes with blunt or rounded apical spines (pointed in N. ramirezi ), and setae shorter than in N. ramirezi .
Apterous viviparous females of N. staryi (diploid chromosome number 14) and N. michelbacheri (unknown karyotype) are unknown, and it could be that they resemble those of the new species. Alatoid nymphs of both species have antennae shorter than body (in three apterous nymphs of the new species antennae are 0.9–1.3 times body). In addition, setae of processes are inconspicuous in N. staryi nymphs and enlarged at apex in N. michelbacheri nymphs.
N. ramirezi sp. n. can be distinguished from the other species of the nominotypical subgenus by the features of first instar nymphs (or embryos): in this species finger-shaped processes are approximately 4–5 times longer than width at middle, as in N. edwardsi , in all other species they are much shorter (see drawing in Quednau, 2010), but these processes in N. edwardsi have shorter apical spines and relatively longer terminal setae.
Although we were unable to obtain sequences from the less variable tRNA-COII locus, the more variable COI barcode locus separates this species from the other Neuquenaphis species sampled, well beyond the 1–2% minimum distance generally discriminating the most closely related aphid species ( Foottit et al., 2008).
The identification of apterous viviparous females of N. ramirezi sp. n. and also of N. blackmani sp. n. and N. aurata sp. n., collected in the future can be done using the dichotomous key below, which has been built on the basis of the identification keys for the species of the subgenus Neuquenaphis by Quednau (2010). It is advisable to use Quednau’s and Blackman & Eastop’s (2018) keys to corroborate future identifications, especially if the resolution of some couplets is difficult.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Spicaphidinae |
|
Genus |