Terebellides stroemii Sars, 1853 sensu Holthe (1986a)
|
publication ID |
https://doi.org/ 10.5281/zenodo.202357 |
|
DOI |
https://doi.org/10.5281/zenodo.5667033 |
|
persistent identifier |
https://treatment.plazi.org/id/8F4F706B-FFC5-EC5A-FF41-09E49C23FADA |
|
treatment provided by |
Plazi |
|
scientific name |
Terebellides stroemii Sars, 1853 sensu Holthe (1986a) |
| status |
|
Terebellides stroemii Sars, 1853 sensu Holthe (1986a) View in CoL
Figures 11 View FIGURE 11 , 12 View FIGURE 12 , 13 View FIGURE 13 d
Terebellides stroemii Sars, 1853 View in CoL 396. Garraffoni et al. 2005: 14. Garraffoni and Lana 2003: 356. Garraffoni and Lana 2004: 973, Tables 1 View TABLE 1 –2, figs. 5–6. Jirkov 2001: 529, figs. 1–5.
Terebellides stroemi Williams 1984: 119 View in CoL , figs. 1 a–c, 3, 6. Imajima and Williams 1985: 11. Holthe 1986a: 170. Solís-Weiss et al. 1981: 147. Bremec and Elías 1999: 177.
Material examined. A total of 893 specimens (28.71% of total) were obtained in 185 BIOICE samples. BIOICE sample 2065 (one specimen in one SEM stub IMNH 24933; 66º41'88''N; 20º02'98''W, 148 m). BIOICE sample 2152 (six specimens in three SEM stubs IMNH 24934 to IMNH 24936; 66º08'91''N; 17º35'85''W, 198 m).
Additional material examined. Naturhistorisk Museum, Universitetet i Oslo, C-1528. Bundefjord (Kr.ania), 45–50 fr., 15.09.1908 (Wollebaeck det.). Two vials with 68 and 38 specimens respectively; one vial revised by A. Wollebaek in 1908 and one by T. Holthe in 1975.
Occurrence. Present in a wide range of depths and temperatures, both in cold northern and warm southern waters. Depth range: 173−1951 m; temperature range: -0.8ºC to 7.2ºC.
Distribution. Since Williams (1984), the cosmopolitan status of T. stroemii has been questioned, and its distribution is now thought to be limited to Boreo-Arctic waters of the Atlantic Ocean ( Hutchings & Peart 2000). This species was previously reported in Icelandic waters by Wesenberg-Lund (1951).
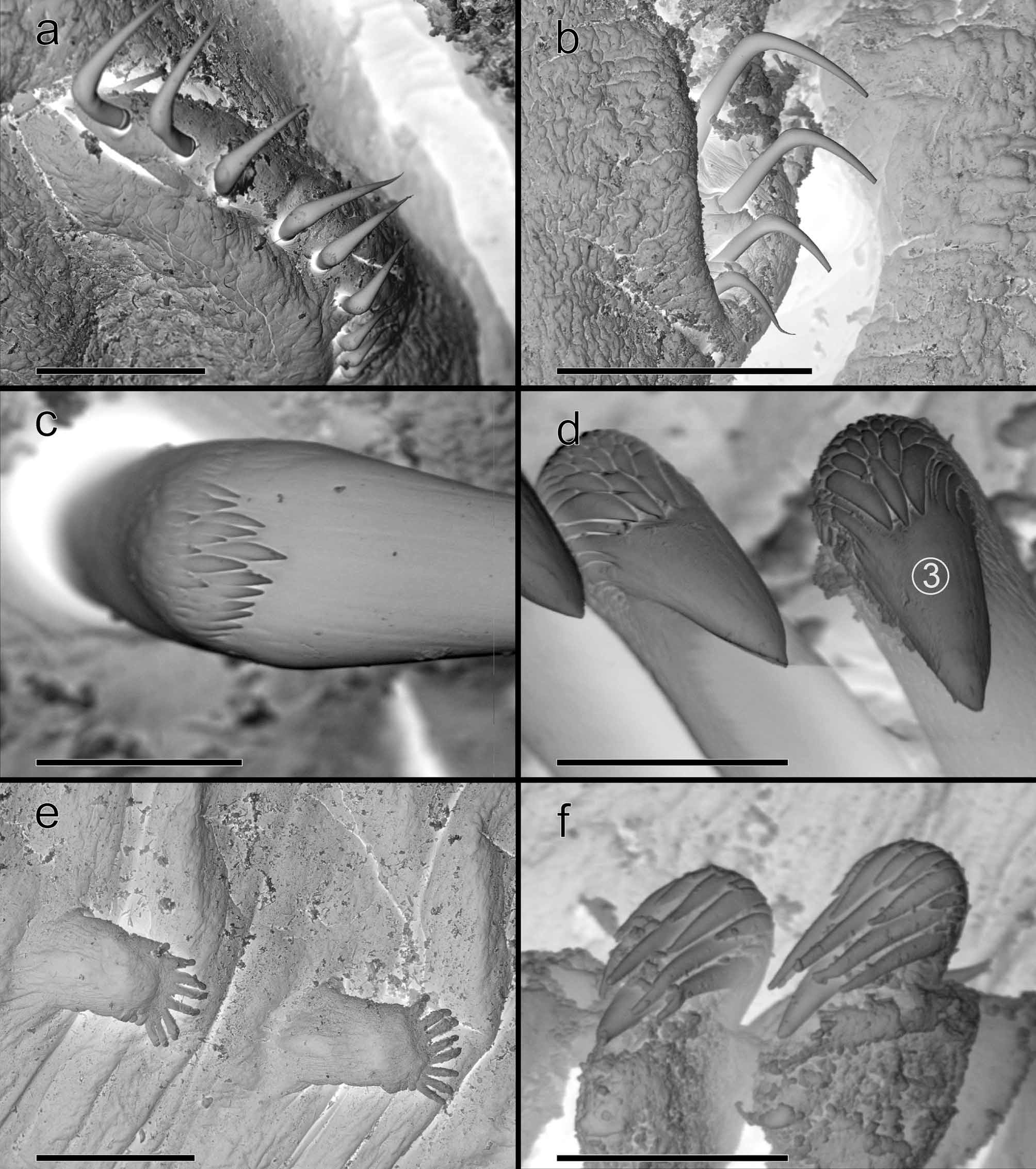
Remarks. The characteristics of the Icelandic specimens correspond fairly well with the expanded redescription of specimens of T. stroemii from Norway provided by Hutchings and Peart (2000). Lateral lappets are present on segments 3–7 declining in size posteriorly and forming dorsal rounded projections on the same segments ( Fig. 11 View FIGURE 11 a–c). However, their development varies among the specimens, being usually larger on segments 4–6 (chaetigers 2–4) and particularly on the fifth ( Fig. 11 View FIGURE 11 c). These differences may be due to age or sexual maturity so this character should be used with caution in the characterization of the species. Branchiae consist of a typical mid-dorsal stalked structure made up of four lobes fused for half of their length ( Fig. 11 View FIGURE 11 d) and provided with numerous tightly packed flat lamellae with well defined concentric rows of cilia ( Fig. 11 View FIGURE 11 e). However, the Icelandic material differs from the Norwegian specimens as redescribed by Hutchings and Peart (2000) in two aspects: the position of nephridial papillae and the shape of the acicular chaetae. Thus, nephridial papillae are located in segments 6–7 (chaetigers 4–5) in BIOICE specimens ( Fig. 11 View FIGURE 11 b, f) instead of segments 3–4 ( Hutchings & Peart 2000; fig. 16a), and the first thoracic neuropodia of segment 8 (chaetiger 6) are sharply bent (90º) geniculate chaetae with pointed tip in BIOICE specimens ( Fig. 12 View FIGURE 12 a–b) instead of gently curved with blunt tip ( Hutchings & Peart 2000; fig. 16b). The study of specimens from Bundefjord (West Norway), identified as T. stroemii by A. Wollebaeck and T. Holthe, revealed that the arrangement of the nephridial papillae on segments 6 and 7 is consistent with that of BIOICE specimens. However, Holthe also identifies a first nephridial pore in segment 3 (chaetiger 1), which we detected neither in the Icelandic nor in the Norwegian material. This suggests that neither the material we examined nor the specimens studied by Hutchings and Peart (2000) would correspond to T. stroemii . It is very likely, according to Hutchings and Peart (2000) and contrary to what Holthe (1986a) pointed out, that T. stroemii represents a number of closely related species. Perhaps a future revision of this taxon using other characters, such as distribution of nephridial papillae, can reveal its true diversity.
Acicular chaetae from BIOICE specimens also have denticles on their apical end forming a capitium ( Fig. 12 View FIGURE 12 c). Thoracic uncini are characterized by a group of three large teeth over the main tooth (dental formula: MF:3:4) ( Fig. 12 View FIGURE 12 d) while the abdominal ones follow the general trend of these uncini in other species ( Fig. 12 View FIGURE 12 e–f).
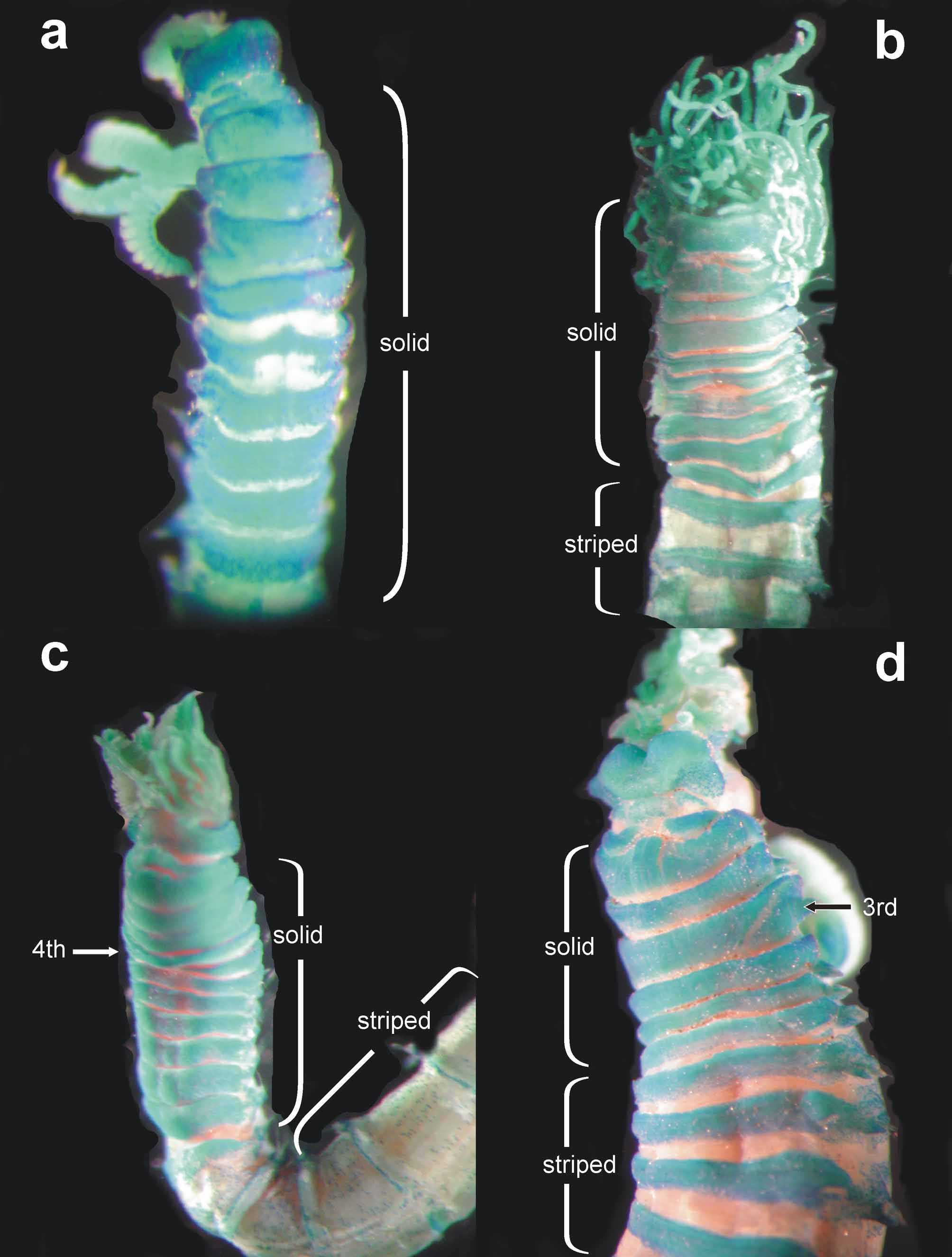
The MG staining ( Fig. 13 View FIGURE 13 d) results in a compact green coloration of the first 8 segments (six chaetigers), after turning into a striped pattern and fading in the posterior thoracic segments. The oval-shaped glandular region of segment 5 (chaetiger 3) is also stained.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Terebellides stroemii Sars, 1853 sensu Holthe (1986a)
| Parapar, Julio, Moreira, Juan & Helgason, Gudmundur V. 2011 |
Terebellides stroemii
| Garraffoni 2005: 14 |
| Garraffoni 2004: 973 |
| Garraffoni 2003: 356 |
| Jirkov 2001: 529 |
Terebellides stroemi
| Bremec 1999: 177 |
| Holthe 1986: 170 |
| Imajima 1985: 11 |
| Williams 1984: 119 |