Ceriodaphnia smirnovi, Alonso & Neretina & Ventura, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4974.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:13607C49-59EA-4170-B45B-876F9CA8F87B |
|
DOI |
https://doi.org/10.5281/zenodo.4916630 |
|
persistent identifier |
https://treatment.plazi.org/id/874087C9-061A-FB21-ABFA-CC4DFBA6FD77 |
|
treatment provided by |
Plazi |
|
scientific name |
Ceriodaphnia smirnovi |
| status |
sp. nov. |
Ceriodaphnia smirnovi sp. nov.
( Figs. 4–13 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 )
Ceriodaphnia quadrangula View in CoL .— Alonso 1996: 196–198, figs. 86–87.
Ceriodaphnia cf. quadrangula View in CoL .— Ghaouaci et al. 2018: 416.
Ceriodaphnia sp. — Marrone et al. 2019: 174, tab. 2.
? Ceriodaphnia quadrangula View in CoL .— Brehm 1954: 339.
Etymology. This new species is named after Prof. Nikolai N. Smirnov who passed away recently. He was Principal Scientist of the Laboratory for Ecology of Aquatic Communities and Invasions (A.N. Severtsov Institute of Ecology and Evolution,the Russian Academy of Sciences).He made a significant contribution to the development of zoological studies in the USSR and Russia and had a significant impact on zooplankton research in other countries.
Type locality. Lucio del Cangrejo , Doñana National Park ( 37.031586°N; 6.276917°W), Huelva, Spain, coll. M. Alonso, January 2019 GoogleMaps .
Type material. Holotype. Adult ephippial female in 4% formaldehyde deposited at the collection of MNCN (accession number: 20.04/12449). The label of holotype is: “ Ceriodaphnia smirnovi sp. nov., 1 eph. ♀. Lucio del Cangrejo , Parque Nacional Doñana , Huelva, España, Holotipo ”.
Allotype. Adult male in4%formaldehyde deposited at the collection of MNCN (accession number:20.04/12450). The label of allotype is: “ Ceriodaphnia smirnovi sp. nov., 1 ♂. Lucio del Cangrejo , Parque Nacional Doñana , Huelva, España, Alotipo ”.
Paratypes. Nine undissected parthenogenetic females, 10 undissected males,10 undissected ephippial females in 4% formaldehyde deposited at the collection of MNCN (accession numbers: 20.04/12451-20.04/12479). The label of the paratypes is: “ Ceriodaphnia smirnovi sp. nov. Lucio del Cangrejo, Parque Nacional Doñana, Huelva, España, Paratipos” .
Other material studied. Thirty parthenogenetic females, 25 ephippial females and 15 males from a peanut field with sandy pools El Frine 1 ( 36.838153°N; 8.422069°E), Algeria, coll. S. Ghaouaci & M. Amarouayache, 18.01.2014, AAK 2016-007; GoogleMaps 10 parthenogenetic females, 5 ephippial females, 2 males from Pozza di Buccheri ( 37.11994°N; 14.8554°E), Sicily, Italy, coll. F. Marrone, 03.04.2010, AAK M-5311; GoogleMaps 10 parthenogenetic females, 10 ephippial females, 10 males from Margio di Anguillara ( 37.85847°N; 12.92082°E), Sicily, Italy, coll. F. Marrone, 02.03.2014, AAK M-5314 GoogleMaps .
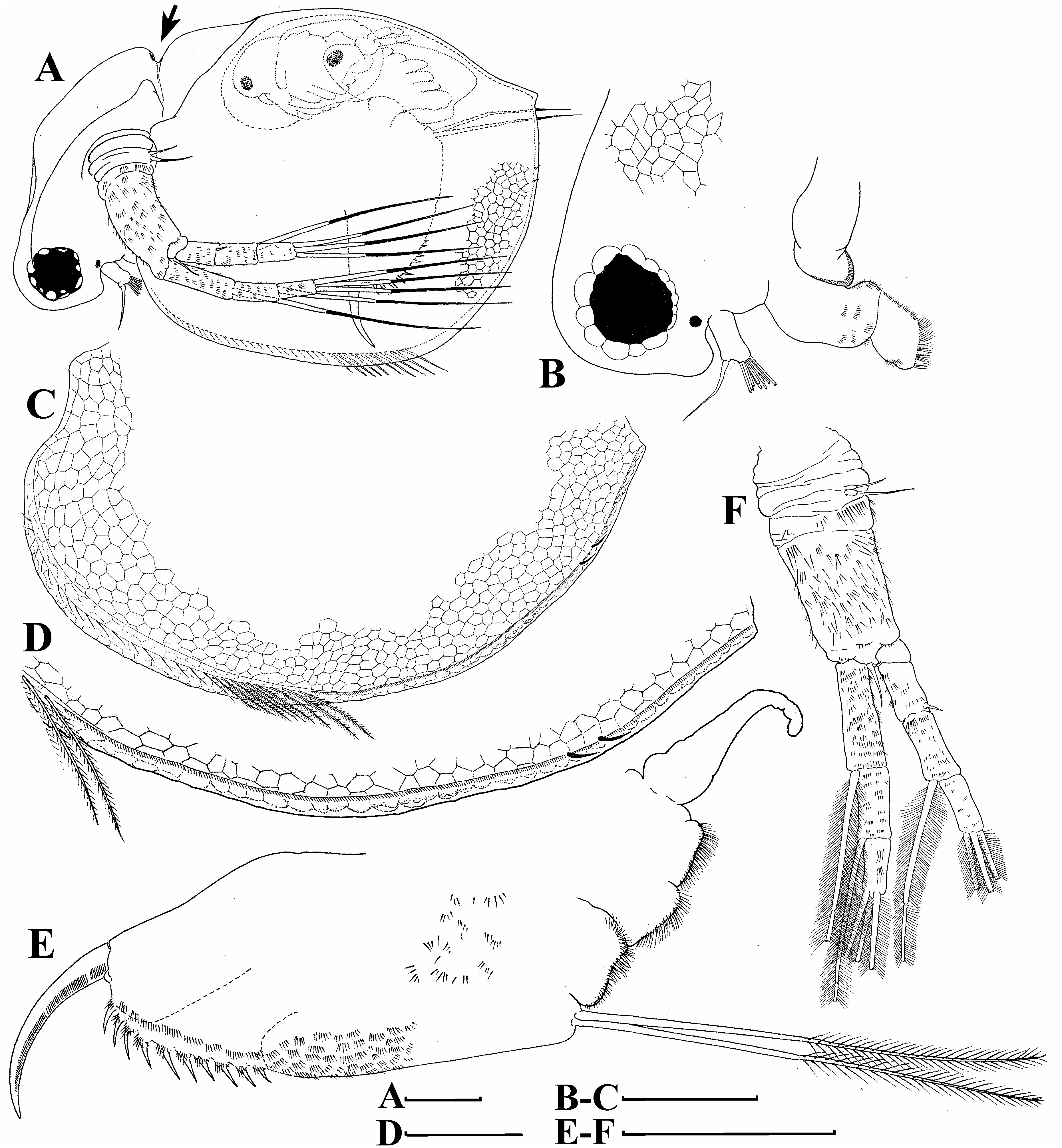
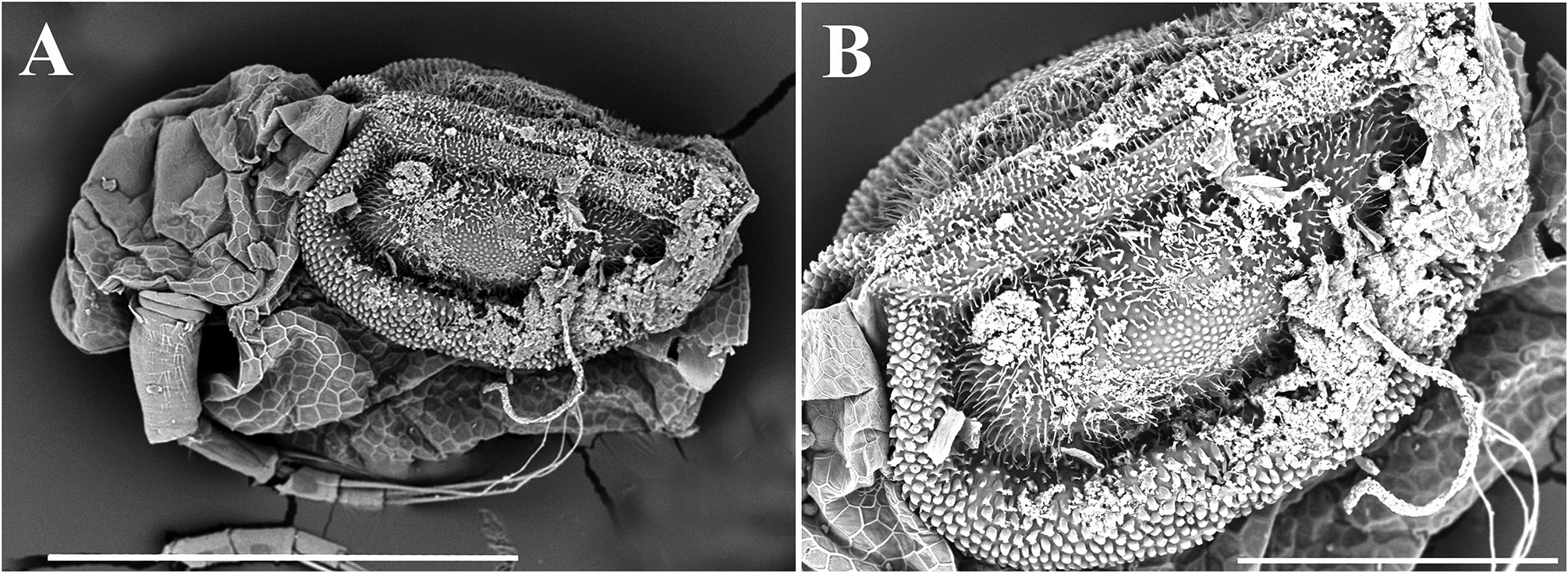
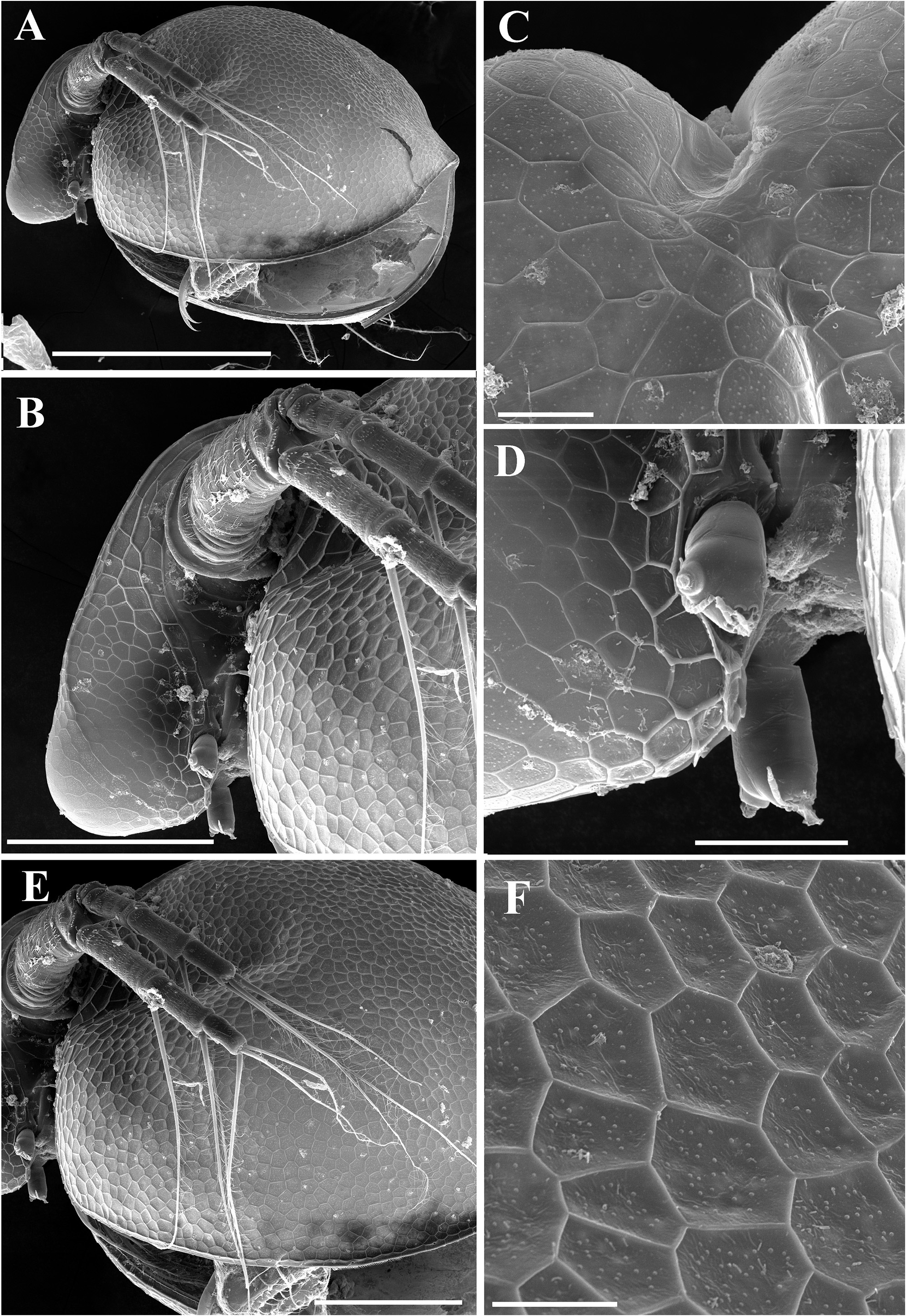
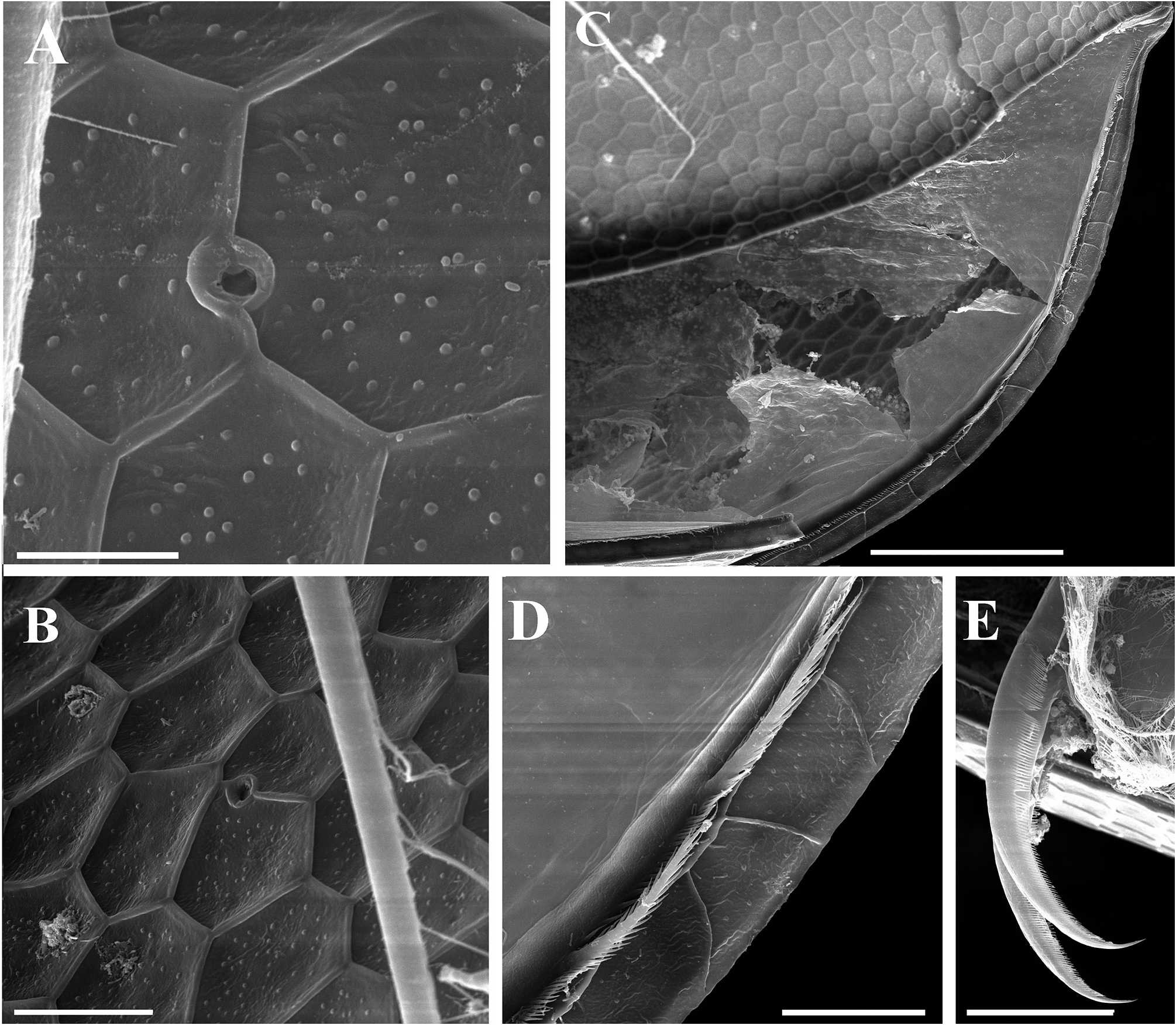
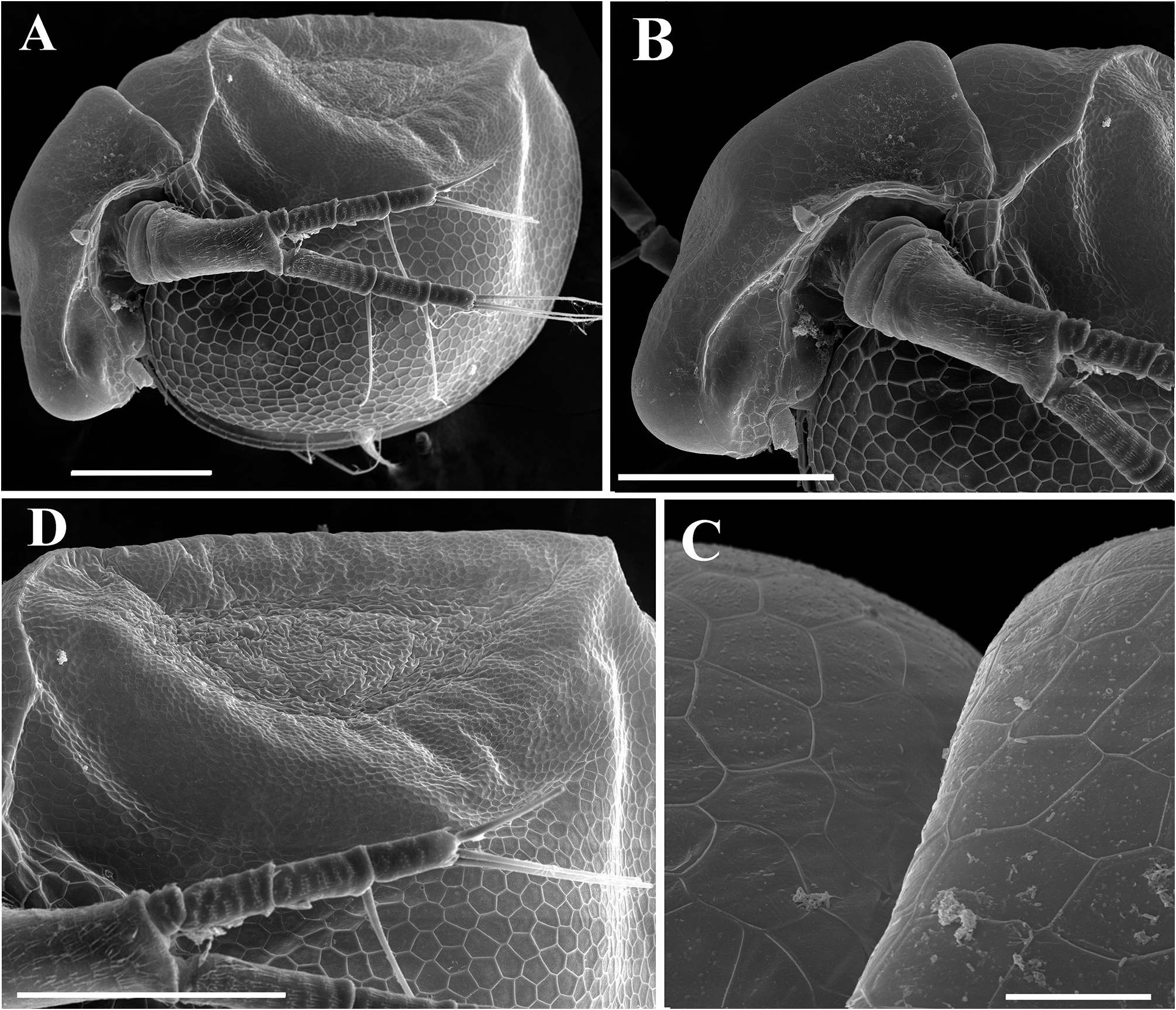
Description. Parthenogenetic female. General ( Figs. 4A View FIGURE 4 , 8A View FIGURE 8 ). Body rounded in lateral view, typical for genus (body height/length=0.70), maximum height in middle portion. Dorsum of valves significantly elevated under head, broadly convex, with prominent dorsolateral depression between head and rest of body ( Figs. 4A View FIGURE 4 , 8A View FIGURE 8 ). Posterodorsal angle well visible. Ventral margin broadly rounded, smoothly passing to anteroventral margin. Very prominent sculpture on body represented by polygons. Each polygon with tiny warts ( Figs. 8F View FIGURE 8 , 9A–B View FIGURE 9 ). No integumental setae on head and valves ( Figs. 8B, E View FIGURE 8 ). Small pseudopores present on valves ( Figs. 9A–B View FIGURE 9 ) and on head shield at level of antenna II. Body laterally compressed, elongated and subovoid in dorsal and ventral view.
Head ( Figs. 4A–B View FIGURE 4 , 8A–D View FIGURE 8 ) small, with prominent rounded rostrum and voluminous supraocular dome surrounding large compound eye ( Figs. 4A–B View FIGURE 4 ). Posterior margin of dome forming supraocular depression. Minute ocellus rounded, located near base of antenna I ( Figs. 4A–B View FIGURE 4 ). Frontal head pore not revealed. Dorsal head pore round, prominent, located very close to dorsal head depression ( Fig. 4A View FIGURE 4 ). Ornamentation of head is represented by polygons with tiny warts ( Figs. 8B–D View FIGURE 8 ).
Labrum ( Fig. 4B View FIGURE 4 ) with wide, fleshy main body and large, setulated distal labral plate strongly compressed laterally.
Valves ( Figs. 4C–D View FIGURE 4 , 8E–F View FIGURE 8 , 9A–D View FIGURE 9 ) large, almost rounded. On inner side, anteroventral margin with row of short setae followed by short group of longer setae in ventral margin ( Figs. 4C–D View FIGURE 4 ), posteriorly this row represented by only fine setulae until last one-third of ventral margin where two or three short plumose setae appear ( Figs. 4C–D View FIGURE 4 , 9D View FIGURE 9 ). Caudal spine and lateral protuberances absent ( Figs. 4A, C View FIGURE 4 , 8A View FIGURE 8 ).
Thorax relatively long, abdomen short, with single abdominal projection ( Fig. 4A View FIGURE 4 ).
Postabdomen ( Figs. 4E View FIGURE 4 , 9E View FIGURE 9 ) elongated, subrectangular. Ventral margin almost straight or slightly concave. Large anus located closer to base of postabdominal claws. Preanal margin long, slightly concave or straight. Anal margin two times shorter than preanal margin. Postanal margin very short, typical for Ceriodaphnia . Preanal and anal margins with eight to ten pairs of sharp denticles approximately as long as thickness of base of postabdominal claw. Base of each denticle covered by fine setulae. Also, row of clusters of fine setulae located above row of denticles on anal portion and gradually continuing to preanal portion. Small additional clusters of setulae on dorsal and lateral sides of preanal portion.
Postabdominal seta ( Fig. 4E View FIGURE 4 ) as long as postabdomen.
Postabdominal claw ( Figs. 4E View FIGURE 4 , 9E View FIGURE 9 ) massive, slightly curved, with particularly sharp, pointed tip. Lateral side on ventral margin with row of fine denticles on outer face and row of fine denticles on inner face ( Fig. 9E View FIGURE 9 ). Denticles of both rows decreasing in size distally.
Antenna I ( Figs. 4A–B View FIGURE 4 , 8B, D View FIGURE 8 ) cylindrical, thin, short (length about 2.5 diameters), without prominent rows of setulae.Antennular sensory seta slender, subequal in length to antenna I, arising subdistally on inflated protuberance. Nine short aesthetascs subequal in size ( Figs. 4A–B View FIGURE 4 , 8B, D View FIGURE 8 ).
Antenna II long ( Figs. 4A, F View FIGURE 4 , 8A, E View FIGURE 8 ). Coxal part with two sensory setae of different length. Basal segment robust, with thin distal seta on anterior face and relatively short distal sensory seta on posterior face. Basal segment covered by transverse rows of setae. Antennal branches elongated, four-segmented exopod is slightly shorter than three-segmented endopod, all segments cylindrical, covered by rows of short setulae, in some cases, relatively long setae. Antennal formula: setae 0-0-1-3/1-1-3, spines 0-1-0-0/0-0-0. Both exopod and endopod branches with three long, apical swimming setae, all with basal and distal segments bilaterally feathered by fine, long setulae. Lateral setae of exopod and endopod with same armature. Spine on second exopod segment short, thin. Apical spines absent.
Thoracic limbs: five pairs ( Figs. 5A–E View FIGURE 5 ).
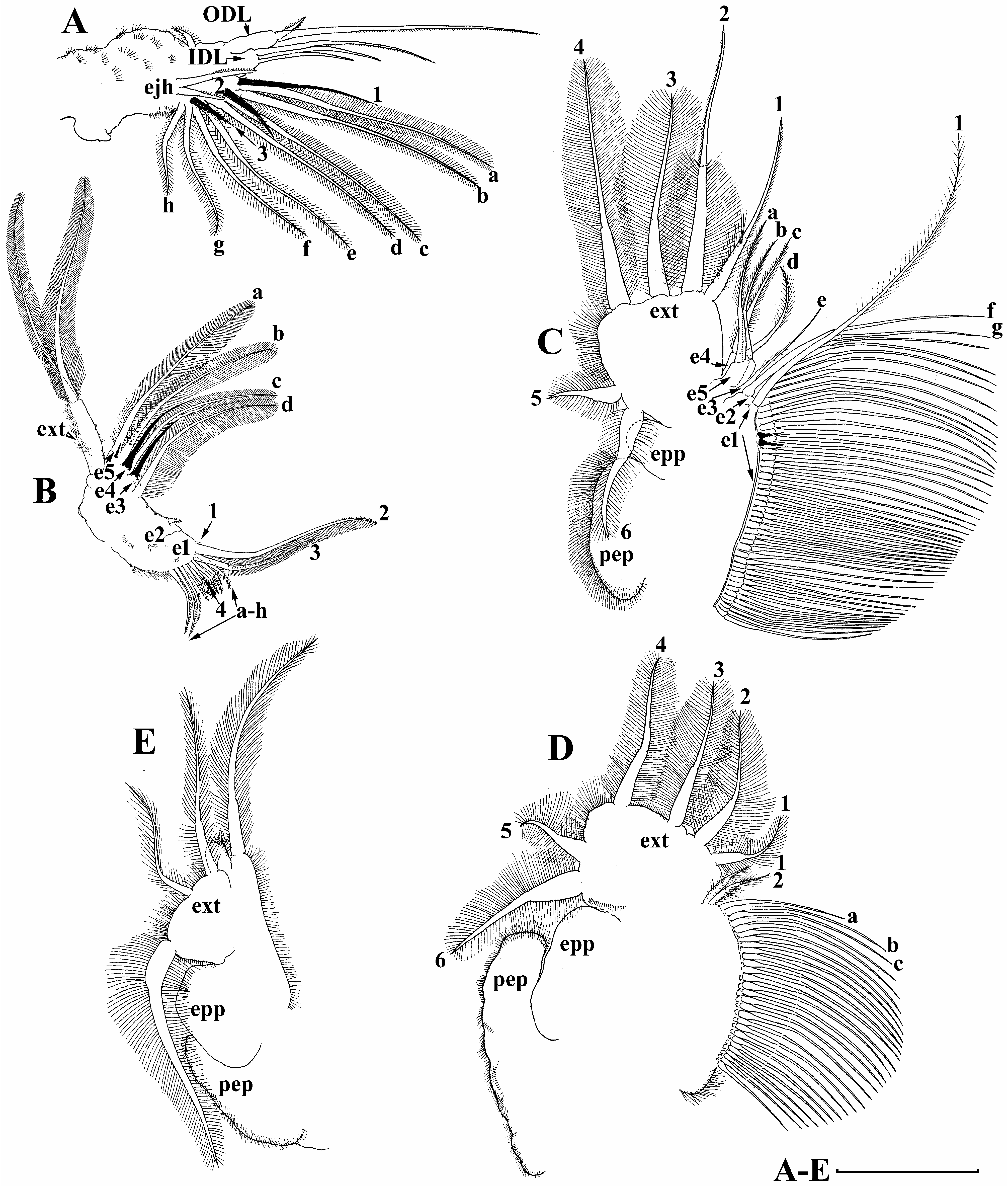
Limb I ( Fig. 5A View FIGURE 5 ) with elongated corm; outer distal lobe with long seta, unilaterally armed distally with short setulae and short thin seta. Inner distal lobe (or endite 5 sensu Kotov 2013), with three unequal setae armed with short stiff setulae. Endite 4 with single anterior seta ( Fig. 5A View FIGURE 5 :1) and two posterior soft setae ( Fig. 5A View FIGURE 5 : a–b). Endite 3 with single anterior seta ( Fig. 5A View FIGURE 5 : 2) and two posterior soft setae ( Fig. 5A View FIGURE 5 : c–d). Endite 2 with single short anterior seta ( Fig. 5A View FIGURE 5 : 3) and four posterior soft setae ( Fig. 5A View FIGURE 5 : e–h). Two ejector hooks of different size. No maxillar process on limb base ( Fig. 5A View FIGURE 5 ).
Limb II ( Fig. 5B View FIGURE 5 ) large. Limb distal portion (exopodite) as large elongated lobe with two soft unequal setae. Endite 5 with single very small anterior stiff seta (represented by small sensillum) and two posterior soft setae ( Fig. 5B View FIGURE 5 : a–b). Endite 4 with single very long anterior stiff seta and single soft posterior seta ( Fig. 5B View FIGURE 5 : c). Endite 3 with single stiff anterior seta. Endite 2 with single soft seta ( Fig. 5B View FIGURE 5 : d). Gnathobase (or endite 1) with two clear rows of setae. Anterior row represented by four elements ( Fig. 5B View FIGURE 5 : 1–4), posterior row consisting of eight setae ( Figs. 5B View FIGURE 5 :a–h).
Limb III ( Fig. 5C View FIGURE 5 ) with large setulated subovoid preepipodite and ovoid epipodite. Exopodite flat, with two lateral setae ( Fig 5C View FIGURE 5 : 5–6) and four distal setae ( Fig 5C View FIGURE 5 :1–4). Distal segment of setae 1 and 2 with especially short stiff setulae. Innerdistal portion with five endites. Endite 5 with two setae ( Fig 5C View FIGURE 5 : a–b). Endite 4 with two setae ( Fig 5C View FIGURE 5 : c–d). Endite 3 with single seta ( Fig 5C View FIGURE 5 : e). Endite 2 with two long setae ( Fig 5C View FIGURE 5 : f–g). Remainder of limb inner portion (endite 1) as a singular large lobe, with single anterior seta ( Fig 5C View FIGURE 5 : 1), two anterior sensillae and numerous posterior setae with short stiff setulae.
Limb IV ( Fig. 5D View FIGURE 5 ) with large setulated ovoid preepipodite and subovoid epipodite. Exopodite flat, broadly rounded with two lateral soft setae ( Fig. 5D View FIGURE 5 : 5–6) and four distal soft setae ( Fig. 5D View FIGURE 5 : 1–4). Inner distal portion of limb not subdivided into endites, distally with two short setae of unclear homology ( Fig. 5D View FIGURE 5 : a–b). Most of limb inner margin a gnathobase filter plate consisting of numerous posterior long setae.
Limb V ( Fig. 5E View FIGURE 5 ) with setulated preepipodite and large subovoid epipodite. Exopodite triangular supplied with two long distal setae and especially large lateral seta. Inner limb portion an ovoid flat lobe, with setulated inner margin and single, large seta.
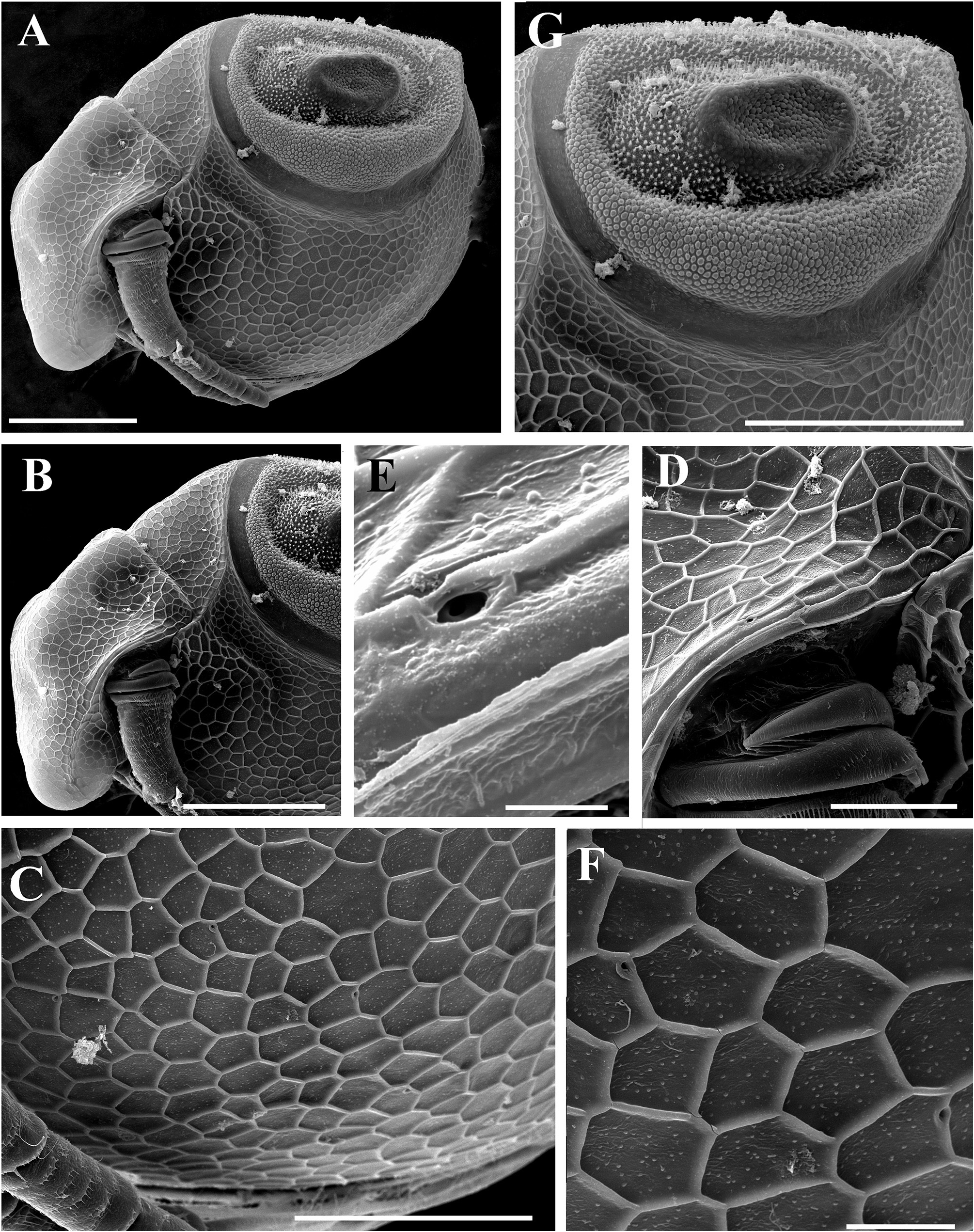
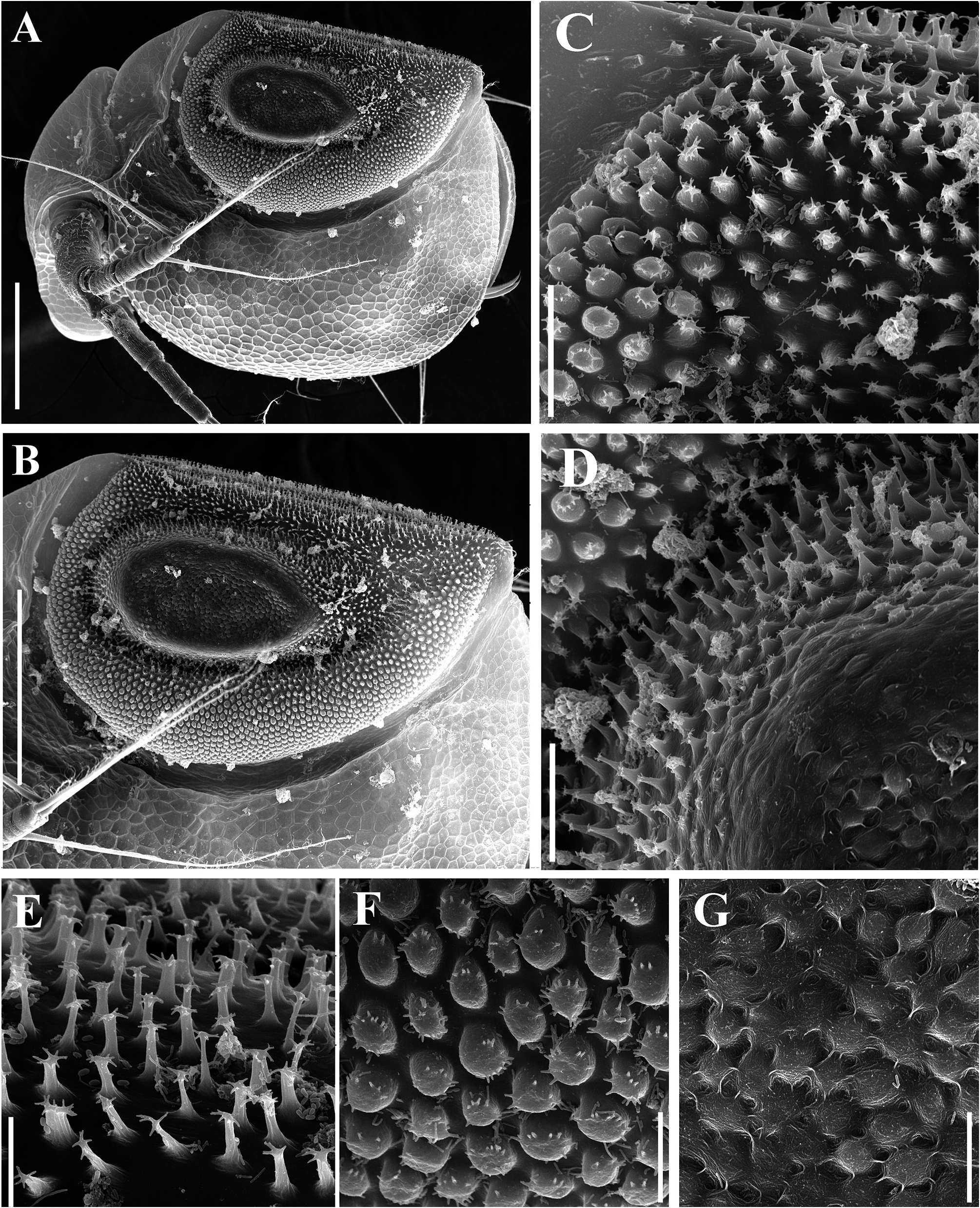
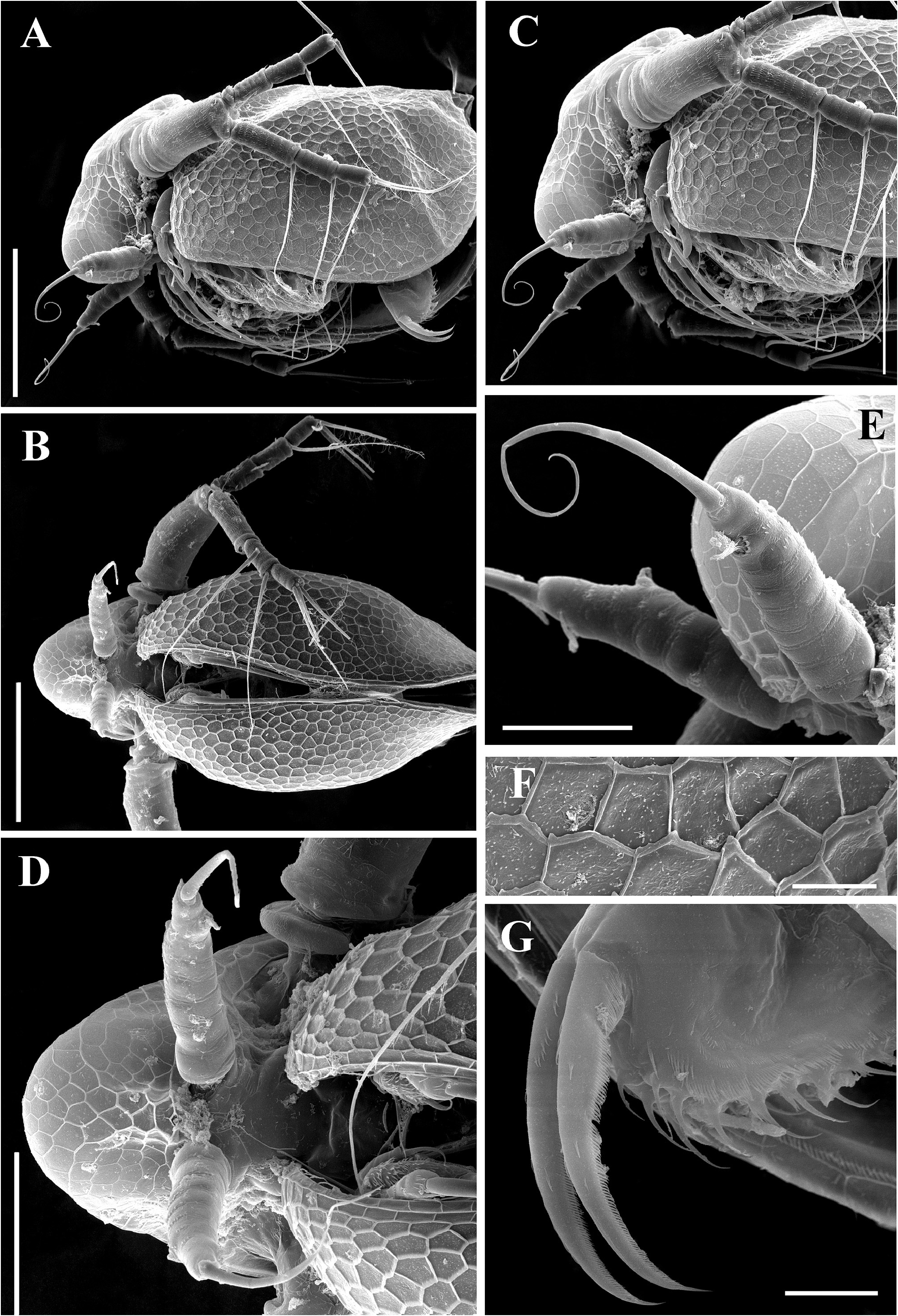
Ephippial female ( Figs. 6A–B View FIGURE 6 , 7A–B View FIGURE 7 , 10A–G View FIGURE 10 , 11A–G View FIGURE 11 ). General body shape and appendages of ephippial female appearing similar to those of parthenogenetic female ( Figs. 6A–B View FIGURE 6 , 7A–B View FIGURE 7 , 10A–G View FIGURE 10 , 11A–G View FIGURE 11 ). Dorsal portion of body in ephippial females transformed into ephippium, bordered from rest of body. In lateral view, ephippium narrowing posteriorly ( Figs. 6A View FIGURE 6 , 7A–B View FIGURE 7 , 10A, G View FIGURE 10 , 11A–B View FIGURE 11 ). Dorsal margin straight; ventral margin regularly curved from posteroventral to anterior margin, almost perpendicular to dorsal margin. Depression along dorsum separating two halves of ephippium well-visible. Sculpture of dorsal portion of ephippium represented by relatively thick small columns with branched tips ( Figs. 6A–B View FIGURE 6 , 7A–B View FIGURE 7 , 10G View FIGURE 10 , 11C View FIGURE 11 ). Egg locule visibly extending laterally ( Figs. 6B View FIGURE 6 , 10A View FIGURE 10 ). Ornamentation of egg locule represented by small columns with branched tips in lateral side and by small tubercles on central portion ( Figs. 11A–G View FIGURE 11 ). Posteroventral, ventral and anterior portions of ephippium covered by small tubercles with short processes. Ephippium containing single resting egg ( Figs. 6A View FIGURE 6 , 10 A, G View FIGURE 10 , 11 A, B View FIGURE 11 ).
Pre-ephippial female ( Figs. 12A–D View FIGURE 12 ). Details of body shape and structure in pre-ephippial female similar to parthenogenetic and ephippial females. In contrast to parthenogenetic female, dorsal portion of valves in preephippial female already different from ventral portion by more fine and delicate reticulation ( Figs. 12A, D View FIGURE 12 ). In contrast to ephippial female, dorsal portion of valves in preephippial female lack any traces of tubercles and processes, although position of future egg locule well visible ( Fig. 12D View FIGURE 12 ).
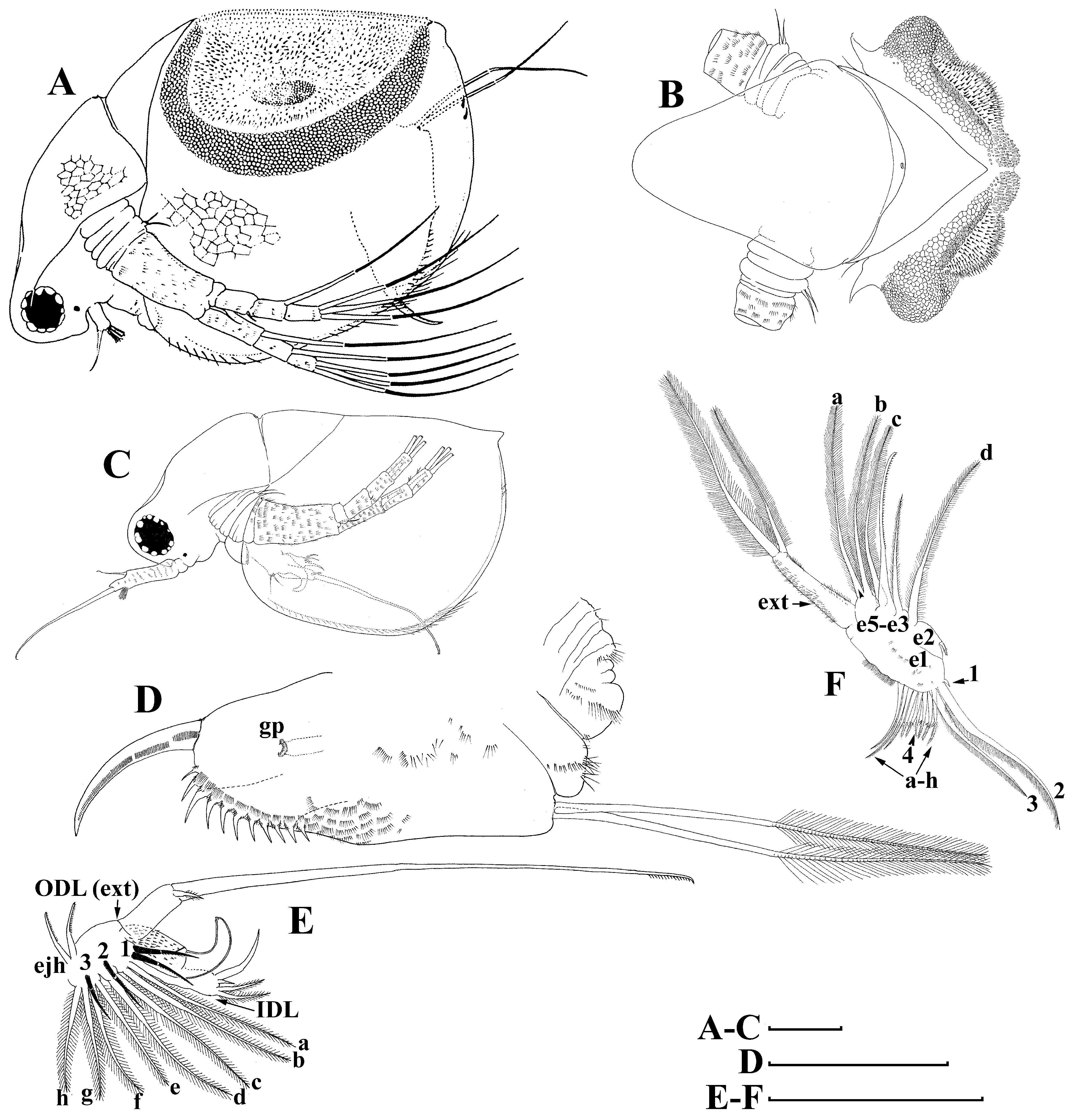
Male ( Figs. 6C–F View FIGURE 6 , 13A–G View FIGURE 13 ). General ( Figs.6C View FIGURE 6 , 13A View FIGURE 13 ). Body ovoid in lateral view, more elongated compared to female (body height/length about 0.56). Dorsal margin of valves not elevated above head, posteroventral angle distinct.
Head ( Figs. 6C View FIGURE 6 , 13A, C View FIGURE 13 ) small, more elongated than in female. Dorsal head pore present ( Fig. 6C View FIGURE 6 ). Compound eye large ( Fig. 6C View FIGURE 6 ). Ocellus rounded or slightly elongated, located near base of antenna I ( Fig. 6C View FIGURE 6 ).
Valves ( Figs. 6C View FIGURE 6 , 13A, C View FIGURE 13 ) ovoid, more elongated than in female. Armature of inner side as in female.
Postabdomen ( Figs. 6D View FIGURE 6 , 13G View FIGURE 13 ) generally as in female. Gonopores ( Fig 6D View FIGURE 6 : gp) open laterally at beginning of distal third of postabdomen.
Antenna I ( Figs. 6C View FIGURE 6 , 13D–E View FIGURE 13 ) long and straight, covered by small stiff setae.Antennular sensory seta long, arising subdistally from antennular body. Male seta two times longer than antennular body. Nine terminal aesthetascs on opposite side to antennular sensory seta.
Limb I ( Fig. 6E View FIGURE 6 ) with large, curved copulatory hook with pointed apex. ODL (exopodite) with two setae, one especially long, bi-segmented, its distal portion covered by fine short denticles. IDL (endite 5) additionally with short seta of unknown homology. Additional seta also located near anterior seta 1. Ejector hooks of same size.
Limb II ( Fig. 6F View FIGURE 6 ) with distal portion (exopodite) similar to female. Endite 5 with single relatively small stiff anterior seta (represented by sensillum) and two soft posterior setae ( Fig. 6F View FIGURE 6 : a–b). Endite 4 with single anterior stiff long seta unilaterally armed distally with short setulae and single posterior soft seta ( Fig. 6F View FIGURE 6 : c). Endite 3 with single medium size soft seta. Endite 2 with single soft seta ( Fig. 6F View FIGURE 6 : d) and small seta of unknown homology. Gnathobase (or endite 1) similar to parthenogenetic female.
Size. Adult parthenogenetic females 0.50–0.99 mm in length; ephippial females up to 0.92 mm in length; adult males 0.50–0.70 mm in length. Juvenile females to 0.50 mm in length. Holotype 0.90 mm in length; allotype 0.68 mm in length.
Variability. No significant variability was found in studied individuals from the Mediterranean region.
Differential diagnosis. In Spain, Ceriodaphnia smirnovi sp. nov. can be easily distinguished from other recorded species ( Alonso 1996) by the presence of dorsal head pore, pseudopores on the body and specific ornamentation of ephippium. Egg locules are significantly expanded laterally and well visible even under light microscope ( Fig. 6B View FIGURE 6 ). Also, thick small columns on the dorsal portion and on the lateral surface of egg locule are visible.
Among European species of Ceriodaphnia studied recently by Kotov et al. (2018), the ephippia of C. smirnovi sp. nov. shares some characteristics with C. quadrangula (O.F. Müller, 1785) , such as the presence of columns bearing branched tips ( Kotov et al. 2018: p. 113, figs. 7a–h), and with C. rotunda (Straus, 1820) sensu Sars, 1862 by the presence of small tubercles with short processes on posteroventral, ventral and anterior portions ( Kotov et al. 2018: p. 111–112, figs. 7a–f). However, combinations of these elements are unique for C. smirnovi sp. nov.
Discrimination of C. smirnovi sp. nov. from other species based on male morphology seems problematic, because there is little reliable information on males for other species of Ceriodaphnia , especially from type localities. However, we expect that morphological features of males will be very useful in the taxonomy of Ceriodaphnia by analogy with daphniids (e.g. Hudec 2010; Kotov 2013) and moinids (e.g. Hudec 2010; Kotov 2013; Alonso et al. 2019).
Identification of C. smirnovi sp. nov. based only on parthenogenetic females may be problematic. Significance of all features used in the standard keys should be reevaluated in the future based on type material. We recommend examining: (1) presence of dorsal head pore; (2) shape and armature of the postabdomen (as in the C. quadrangula species group); (3) proportions of stiff anterior setae on limb II (this feature seems significant based on brief analysis of figures from Alonso 1996). Here we found, that limb II in parthenogenetic female of C. quadrangula bears very short anterior setae on endites 4 and 3, whereas in C. smirnovi sp. nov. these setae are especially long and prominent.
Distribution and ecology. Currently, C. smirnovi sp. nov. is known only from Spain ( Alonso 1996), Algeria ( Ghaouaci et al. 2018), Greece ( Marrone et al. 2019) and Italy. Thus, the distribution of C.smirnovi sp. nov. must be clarified in the future based on analysis of material from more sampling points, although, most likely, it is restricted by the Mediterranean region.
The characteristic habitats of C. smirnovi sp. nov. are shallow permanent and/or temporary lagoons in dry regions. Euryhaline waters, with sufficient salt concentrations are relatively important for C. smirnovi sp. nov., although it also can occur in low mineralized waters. Water can be clear or turbid as a result of suspended clay.
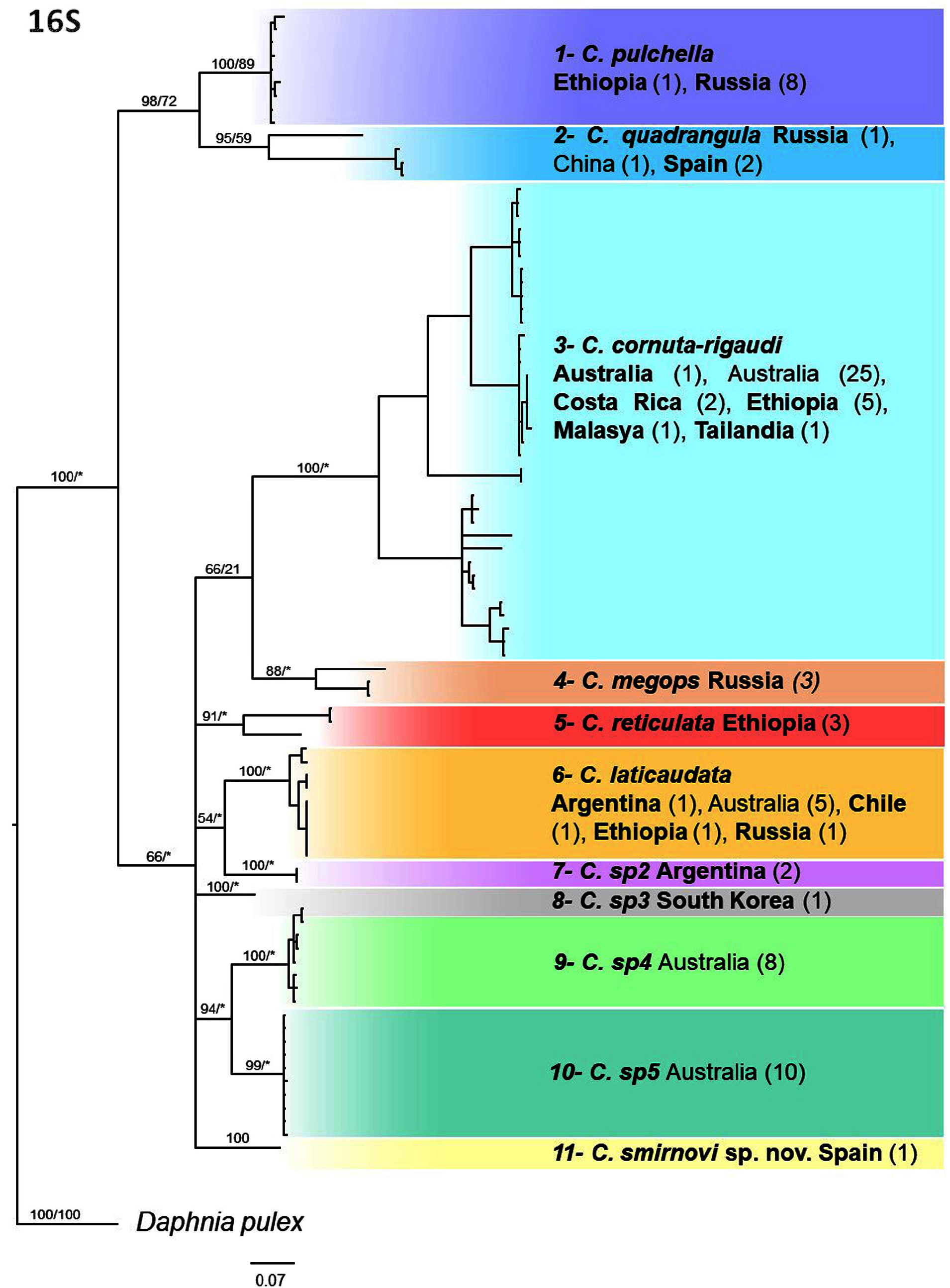
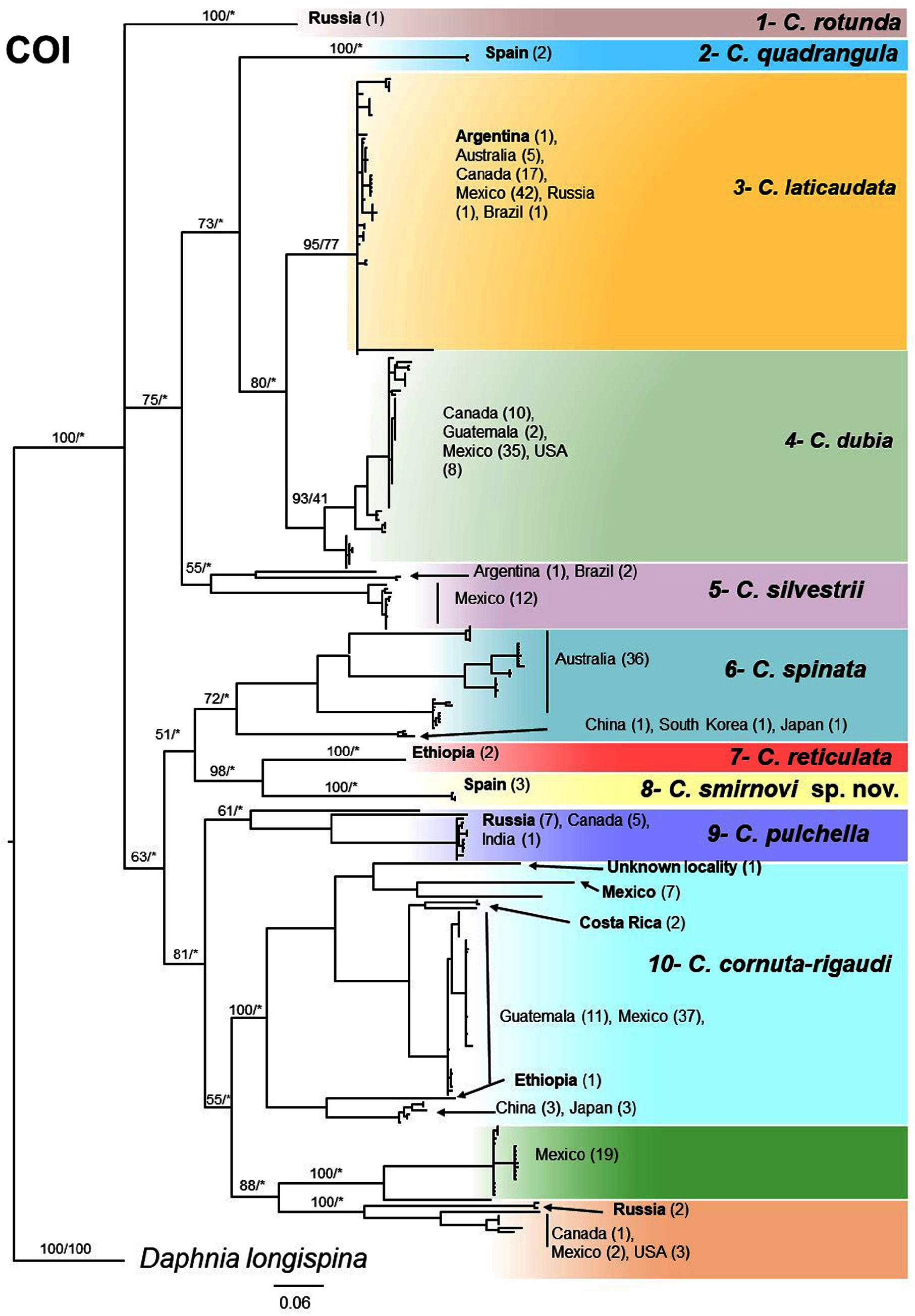
Genetic analyses. From the initially amplified 50 individuals we successfully sequenced 37 individuals for 16S that together with 49 sequences retrieved from GenBank resulted in 11 different clades ( Fig. 14 View FIGURE 14 ), of which 6 correspond to currently described species. We also sequenced 23 individuals for COI that together with 266 sequences retrieved from GenBank resulted in 12 clades ( Fig. 15 View FIGURE 15 ), of which 10 correspond to currently described species. Amongst these 10 species, we were unable to morphologically verify C. dubia , C. spinata and C. silvestrii . In both 16S and COI phylogenetic trees, deep branches had low to moderate support with BI and ML analyses, being not fully concordant within markers or between markers. On the contrary, the distinction of the different clades / species had a very strong support in both phylogenetic trees. The mean genetic distance among the clades was substantial, being 13±2.3% (mean ± SD) for 16S and 20.7±2.1% for COI, while the mean within group distance was 3.6±3.2% and 6.2±3.6%, respectively. Overall we distinguished 16 different lineages, corresponding to 11 described species and to five undescribed species. In addition, five species had large intraspecific variability: C. megops , C. cornuta / C. rigaudi , C. spinata , C. reticulata and C. quadrangula for one or both markers (e.g. genetic distance> 7 % for COI and> 5.6 % for 16S). Also, most of the species had more than one molecular BIN in the BOLD database (overall we found 24 different BINS; Table 2 View TABLE 2 ). This suggests that within most species there are possibly two or more different species and therefore most species included in the phylogenetic analyses of this study should be considered as species groups. Within the 315 sequences retrieved from GenBank we found 7.3% of them which were likely wrongly identified and 28.7% that were not identified that fit within one of the lineages.
| MNCN |
Museo Nacional de Ciencias Naturales |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ceriodaphnia smirnovi
| Alonso, Miguel, Neretina, Anna N. & Ventura, Marc 2021 |
Ceriodaphnia sp.
| Marrone, F. & Alfonso, G. & Stoch, F. & Pieri, V. & Alonso, M. & Dretakis, M. & Naselli-Flores, L. 2019: 174 |
Ceriodaphnia cf. quadrangula
| Ghaouaci, S. & Amarouayache, M. & Sinev, A. Y. & Korovchinsky, N. M. & Kotov, A. A. 2018: 416 |
Ceriodaphnia quadrangula
| Alonso, M. 1996: 196 |