Crassabwa Lugo-Ortiz & McCafferty 1996
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4350.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:A3F42509-91EA-48BC-8C9D-253F5C57F20D |
|
DOI |
https://doi.org/10.5281/zenodo.6045780 |
|
persistent identifier |
https://treatment.plazi.org/id/7C3A878E-4509-5A2F-FF74-FCEAFBCF34A9 |
|
treatment provided by |
Plazi |
|
scientific name |
Crassabwa Lugo-Ortiz & McCafferty 1996 |
| status |
|
Genus Crassabwa Lugo-Ortiz & McCafferty 1996 View in CoL View at ENA
( Figs 1–132 View FIGURES 1 – 4 View FIGURES 5 – 9 View FIGURES 10 – 15 View FIGURES 16 – 19 View FIGURES 20 – 22 View FIGURES 23 – 30 View FIGURES 31 – 36 View FIGURES 37 – 39 View FIGURES 40 – 44 View FIGURES 45 – 50 View FIGURES 51 – 55 View FIGURES 56 – 59 View FIGURES 60 – 65 View FIGURES 66 – 75 View FIGURES 76 – 83 View FIGURES 84 – 95 View FIGURES 96 – 103 View FIGURES 104 – 107 View FIGURES 108 – 113 View FIGURES 114 – 118 View FIGURES 119 – 124 View FIGURES 125 – 132 )
Crassabwa Lugo-Ortiz & McCafferty 1996b: 236 View in CoL . Type species Centroptilum flavum Crass 1947 .
Systematic position of Crassabwa View in CoL . Crassabwa View in CoL belongs to the plesiomorphon Protopatellata Kluge & Novikova 2011. Within this plesiomorphon, it is most closely related to Susua Lugo-Ortiz & McCafferty 1998a View in CoL . Previous descriptions of Susua View in CoL contain errors, which will be corrected in a separate paper. In the present paper, we compare Crassabwa View in CoL with Susua View in CoL based on our examination of Susua niandanense (Wuillot in Wuillot & Gillies 1993b) and Susua sigiense ( Gillies 2001) comb. n. (originally described as Cheleocloeon sigiense View in CoL ). Among characters of Crassabwa View in CoL listed below, setation of larval femur [character (12)] and structure of larval claw [character (19)] are common for Crassabwa View in CoL and Susua View in CoL , and probably represent apomorphies for the lineage comprising these two genera.
Annotated diagnosis of Crassabwa . This diagnosis is based on three species, C. flava , C. ludmilae sp. n. and C. ameliae sp. n. described below. In all three species, last instar larva has peculiar structure of the first pair of tergalii, which allows to distinguish it from all other taxa [see (25)]. Another peculiar larval character is presence of a pair of projections on the anterior margin of tenth tergum [see (28)]. Winged stages can be distinguished from other taxa by shape of hind wing [see (21)] (known for all three species) and presence of inner projection on male unistyliger [see (31)] (known only for C. flava and C. ludmilae sp. n.).
(1) Cuticular coloration of larva. Larvae of all three species of Crassabwa have contrasting cuticular coloration consisted of dark areas (varying from ocher to greenish brown or dark brown) and light blanks (varying from colorless to ocher): Head at most part dark, between eyes with composite blanks corresponding to places of attachment of mandibular muscles ( Figs 3 View FIGURES 1 – 4 , 61 View FIGURES 60 – 65 , 116 View FIGURES 114 – 118 ). Thoracic terga at most part dark, with composite blanks (in C. ludmilae sp. n. and C. ameliae sp. n. blanks of pronotum and mesonotum often with darkening inside – Figs 62 View FIGURES 60 – 65 , 124 View FIGURES 119 – 124 ). Abdominal terga with paired medioanterior and medioposterior sigilla forming contrasting blanks; coloration of terga is either similar on all abdominal segments ( Figs 3 View FIGURES 1 – 4 , 57 View FIGURES 56 – 59 , 60 View FIGURES 60 – 65 , 116 View FIGURES 114 – 118 , 119 View FIGURES 119 – 124 ), or different on different segment ( Figs 1, 2 View FIGURES 1 – 4 , 56, 59 View FIGURES 56 – 59 , 114, 115 View FIGURES 114 – 118 ; Crass 1947: Fig. 21a View FIGURES 20 – 22 ); in most cases cuticular pigmentation on terga VII–VIII is less intensive than on terga III–VI and IX (the same in Sususa). Abdominal sterna either all colorless or poorly colored, or sternum IX partly or completely colored with brown. Legs at most part colorless, with variously expressed diffusive brown markings on femur, proximal part of tibia and proximal part of tarsus ( Fig. 64 View FIGURES 60 – 65 ). Caudalii proximally and distally light or colorless, dark brown at middle ( Figs 1–3 View FIGURES 1 – 4 , 56, 57, 59 View FIGURES 56 – 59 , 60 View FIGURES 60 – 65 , 114–117 View FIGURES 114 – 118 , 119 View FIGURES 119 – 124 ).
Cuticle of fore protoptera of last instar larva has species-specific coloration: in C. flava it has dark lines along longitudinal convex veins and blanks along longitudinal concave veins, without color markings on cross veins ( Fig. 4 View FIGURES 1 – 4 ); in C. ludmilae sp. n. and C. ameliae sp. n. it has dark lines along all longitudinal and cross veins ( Figs 56–58 View FIGURES 56 – 59 , 62 View FIGURES 60 – 65 , 114–116 View FIGURES 114 – 118 ). This coloration occurs on the larval cuticle, so it is retained unchanged during all last larval instar, and does not depend upon hypoderm with veins, which is gradually transformed to subimaginal wing; only shortly before molt to subimago the cuticular coloration of protopteron becomes poorly visible on background of the dark subimaginal wing crumpled inside. This species-specific character is present only in the last larval instar; in previous instars, coloration of protoptera of all three species is the same as in the last instar of C. flava .
(2) Hypodermal coloration of all stages. Larva, subimago and imago has more or less expressed red hypodermal pigmentation, whose color varies from light orange to dark red or nearly black: in larva this coloration is either absent ( Fig. 1 View FIGURES 1 – 4 ), or present as reddish markings visible through cuticle ( Figs 2–3 View FIGURES 1 – 4 ); in subimago this hypodermal coloration is always present, but can be poorly visible through brown subimaginal cuticle; in imago this hypodermal coloration is well visible, so that imagoes of both sexes are intensively colored by orange and red, especially in dorsal half of the body ( Figs 31–34 View FIGURES 31 – 36 , 96–97, 100–101 View FIGURES 96 – 103 ).
In C. flava and C. ludmilae sp. n. cerci of imago have red hypodermal coloration in proximal part ( Fig. 31 View FIGURES 31 – 36 ). In immature larva (including beginning of last larval instar) red hypodermal coloration, if present in some parts of the body, is never present in caudalii ( Fig. 1 View FIGURES 1 – 4 ); during development of last instar larva, when tissues of paracercus start to degenerate, and tissues of cerci start to transform into subimaginal cerci, proximal parts of cerci get red hypodermal coloration ( Figs 2–3 View FIGURES 1 – 4 , 59 View FIGURES 56 – 59 ). In subimago this red hypodermal coloration of proximal parts of cerci is retained, but can be invisible through dark brown subimaginal cuticle of cerci. Imaginal cuticle of cerci is colorless, so red coloration of their proximal parts is well visible ( Figs 31, 32 View FIGURES 31 – 36 , 97, 100, 101 View FIGURES 96 – 103 ).
Winged stages of C. ameliae sp. n. are unknown; a single examined mature male larva ready to molt to subimago, has no red coloration of cerci ( Fig. 121 View FIGURES 119 – 124 ), that allows to conclude that winged stages of this species have no red coloration of cerci (see below).
(3) Larval frons. Bases of antennae are widely separated, frons between them is flat ( Fig. 3 View FIGURES 1 – 4 ) (unlike carinate in some other taxa).
(4) Larval frontal suture. Frontal suture elongate ( Fig. 61 View FIGURES 60 – 65 ).
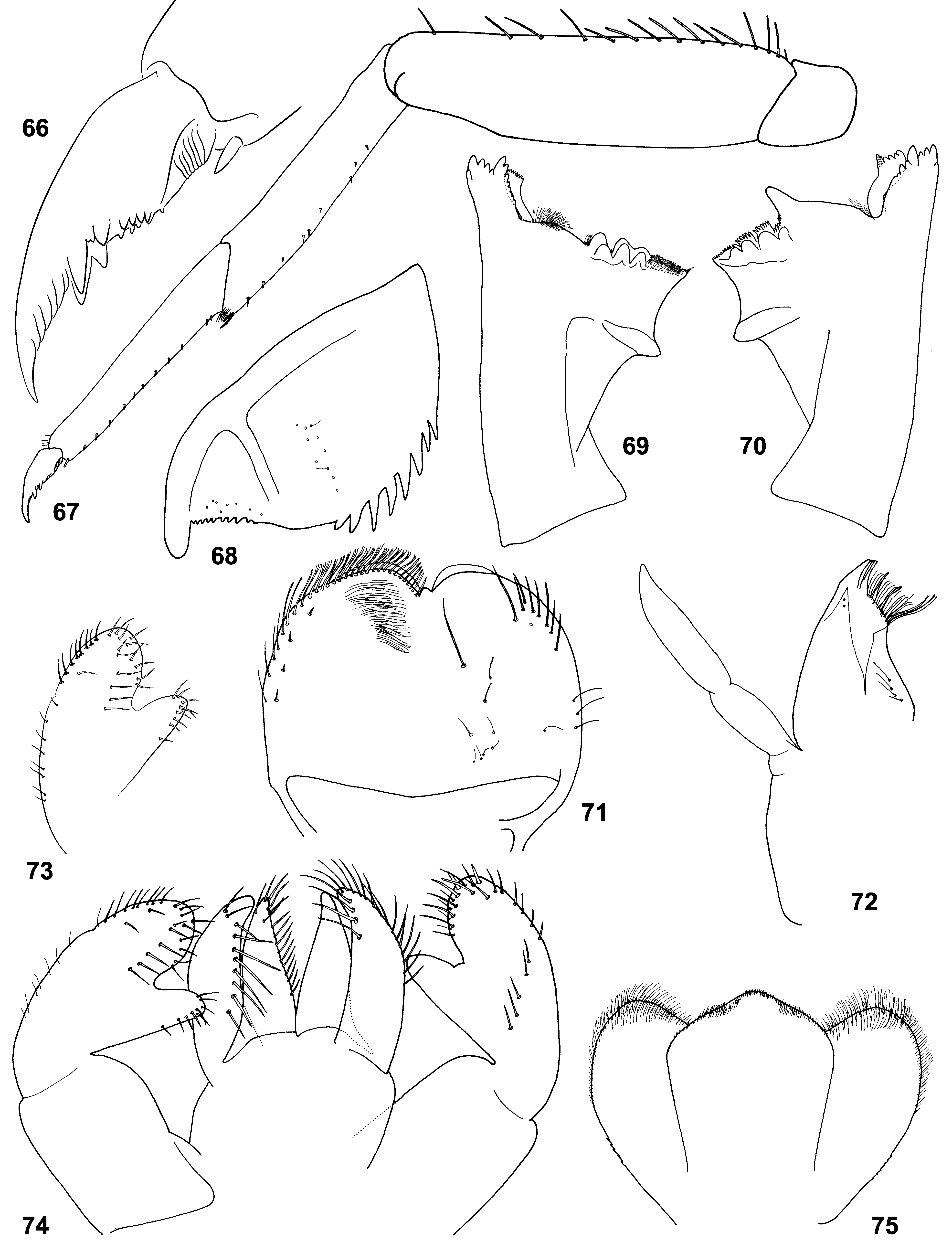
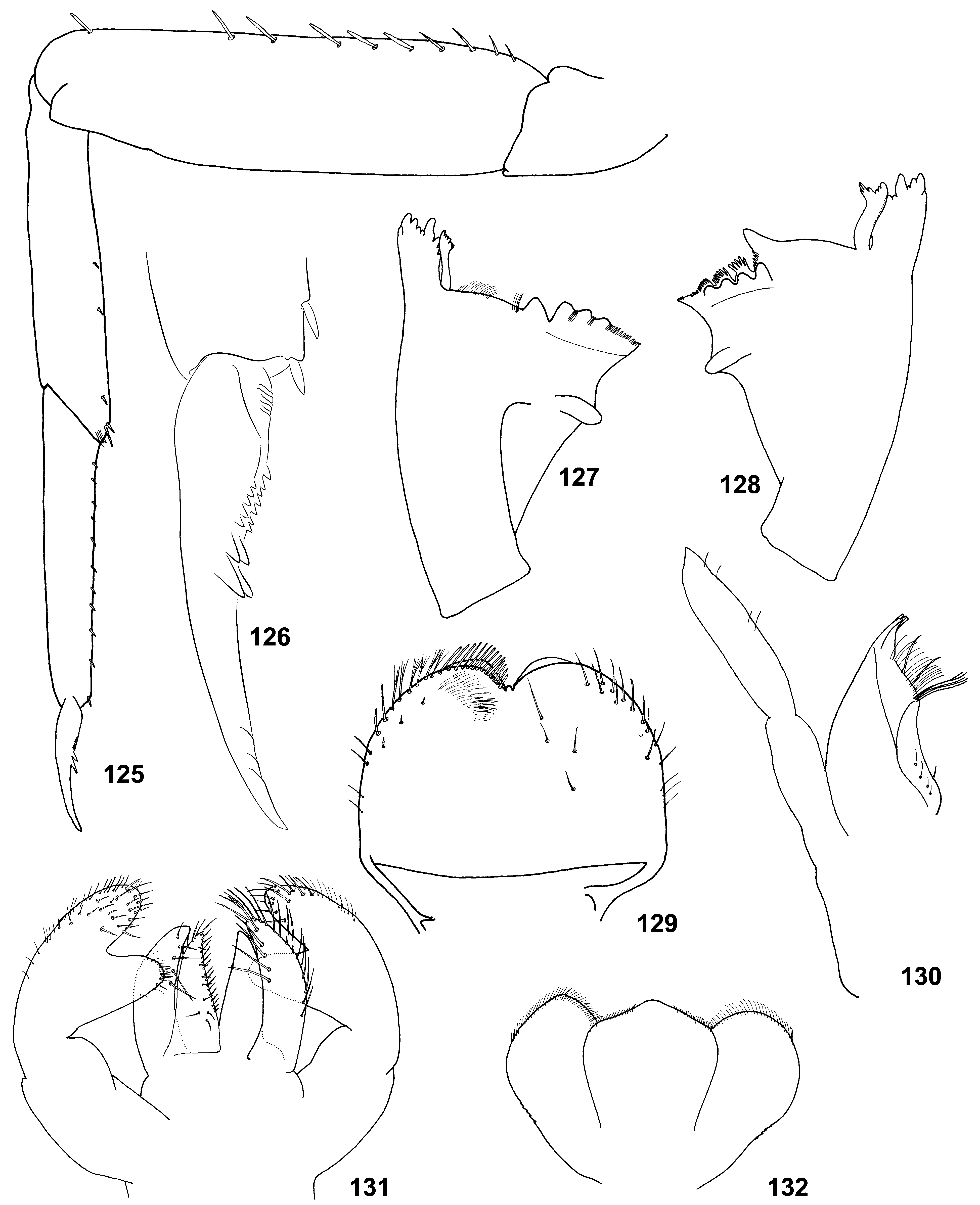
(5) Labrum. Labrum nearly semicircular with median incision; dorsal surface with pair of submedian setae and one or two pairs of latero-distal setae ( Figs 71 View FIGURES 66 – 75 , 129 View FIGURES 125 – 132 ; Lugo-Ortiz & McCafferty 1996b: Fig. 1 View FIGURES 1 – 4 ).
(6) Mandibles. In all three species, incisor and kinetodontium are fused at most length; left prostheca with 4 blunt processes and 3–5 small pointed processes more or less fused together; right prostheca nearly parallel-sided, apically obliquely truncated and divided to several processes terminated at one line ( Figs 8–9 View FIGURES 5 – 9 , 69–70 View FIGURES 66 – 75 , 76–82 View FIGURES 76 – 83 , 127– 128 View FIGURES 125 – 132 ; Crass 1947: Fig. 21f–g View FIGURES 20 – 22 ; Lugo-Ortiz & McCafferty 1996b: Fig. 3–4 View FIGURES 1 – 4 ). In other respects structure of mandibles is species-specific: In C. ludmilae sp. n. and C. ameliae sp. n. structure of molae of both mandibles is markedly different from that of C. flava and other Baetidae (see below). In C. flava and C. ameliae sp. n. setae between prostheca and mola are present only on right mandible ( Figs 8–9 View FIGURES 5 – 9 , 127–128 View FIGURES 125 – 132 ), but in C. ludmilae sp. n. such setae are present on both mandibles ( Figs 69–70 View FIGURES 66 – 75 , 76–82 View FIGURES 76 – 83 ).
In Crassabwa , as in many other taxa, denticles of mandibles are often worn down; in order to study the real shape of mandibles, it is necessary to take a just molted larva; to be absolutely sure that mandibles are not worn down, one can examine mandibles just before molt to the next larval instar ( Figs 8–9 View FIGURES 5 – 9 , 78–79 View FIGURES 76 – 83 ). This examination testifies that right prostheca, whose processes looks as cut off, really has such shape (see Figs 79 and 82 View FIGURES 76 – 83 for comparison).
(7) Maxillae. Maxilla ( Figs 72 View FIGURES 66 – 75 , 130 View FIGURES 125 – 132 ; Lugo-Ortiz & McCafferty 1996b: Fig. 5 View FIGURES 5 – 9 ) has all 3 canines and 3 dentisetae; distal dentiseta much thicker than others, but bent at the same direction as others; among several setae proximad of dentisetae, 1 st one either simple as others ( Fig. 6 View FIGURES 5 – 9 ), or bifid, resembling dentiseta and possibly consists of two setae fused at base ( Fig. 7 View FIGURES 5 – 9 ).
(8) Maxillary palp. Maxillary palp 2-segmented; 2nd segment thick, with short and narrow apical projection ( Figs 72 View FIGURES 66 – 75 , 130 View FIGURES 125 – 132 ; Lugo-Ortiz & McCafferty 1996b: Fig. 5 View FIGURES 5 – 9 ), which possibly represents vestige of 3rd segment.
(9) Labium. Paraglossa slightly wider than glossa ( Figs 5 View FIGURES 5 – 9 , 74 View FIGURES 66 – 75 , 83 View FIGURES 76 – 83 , 131 View FIGURES 125 – 132 ). Setal rows on glossae and paraglossae as in many other taxa: Glossa with following setal rows: median row (regular row of spine-like setae along median margin); dorso-lateral row (regular row along lateral margin, with setal bases visible in dorsal view); ventral side of glossa bears setae directed ventrally, which are located irregularly in the proximal part of the glossa and form a ventro-median row (sparse row parallel to median margin). Paraglossa with following setal rows: latero-apical setae (longest ones) form a regular sparse row along lateral margin, more densely and irregularly located on apical margin, and irregularly located on dorsal side; ventro-median row (regular row of long slender straight setae attached on ventral side and directed mainly medially); dorso-median row (regular row of thicker setae attached on dorsal side and directed medio-distally, limited by distal half of paraglossa).
(10) Labial palp. 2nd segment forms prominent inner-apical projection, which can be markedly thinner than 3rd segment or subequal to it ( Figs 5 View FIGURES 5 – 9 , 73, 74 View FIGURES 66 – 75 , 83 View FIGURES 76 – 83 , 131 View FIGURES 125 – 132 ). 2nd segment contains well-developed muscle-adductor of 3rd segment. Muscle-abductor of 2nd segment is attached at median groove of submentum ( Fig. 5 View FIGURES 5 – 9 ) (in contrast to Susua , where it is attached at base of submentum).
(11) Postsubalar sclerite. Postsubalare has posterior-dorsal corner stretched to a thin process with concave dorsal margin (this is similar to Cloeonini, but differs from Susua and many other taxa, whose posterior-dorsal process is wider, with convex dorsal margin). Postsubalare has the same shape in imago and subimago; it is well visible on subimaginal exuviae due to contrasting cuticular coloration ( Figs 36 View FIGURES 31 – 36 , 98 View FIGURES 96 – 103 ).
(12) Larval femur. Outer margin of each femur bears one row of stout elongate spatulate colorless setae; near apex of femur locates one subapical seta, which has the same structure as other setae of this row, but usually is somewhat shorter and separated from nearest seta by a distance greater than other distances between setae of this row ( Figs 10–13 View FIGURES 10 – 15 , 64 View FIGURES 60 – 65 , 67 View FIGURES 66 – 75 , 122 View FIGURES 119 – 124 , 125 View FIGURES 125 – 132 ; Lugo-Ortiz & McCafferty 1996b: Fig. 7 View FIGURES 5 – 9 ). The same in Susua ; in most other Baetidae larval femur either has two subapical setae brought together, or no recognizable subapical seta. Probably, presence of one subapical seta is a synapomorphy of Crassabwa and Susua .
(13) Patella-tibial suture. In larvae of both sexes and in female at all stages (larva, subimago and imago) fore tibia without patella-tibial suture, middle and hind tibia with patella-tibial suture; in other respects tibiae of all legs have similar structure ( Figs 10–12 View FIGURES 10 – 15 ). This is typical for the plesiomorphon Protopatellata, to which Crassabwa belongs. In larva, patella-tibial suture is stretched far along tibia, so that crosses inner side of tibia at distal part ( Figs 11–12 View FIGURES 10 – 15 , 64 View FIGURES 60 – 65 , 122 View FIGURES 119 – 124 ).
(14) Fine setae on larval tibia. Outer side of tibia near base is crossed by a row of fine colorless setae: portion of this row located on posterior side of tibia is transverse (this transverse portion was erroneously figured on anterior side of tibia by Lugo-Ortiz & McCafferty 1996b: Fig. 7 View FIGURES 5 – 9 ); portion of this row located on anterior side of tibia is oblique, on middle and hind tibia stretching along patella-tibial suture ( Fig. 13 View FIGURES 10 – 15 ); setae of the same kind as in this row, are sparsely and irregularly arranged along posterior side of tibia and tarsus of all legs. In C. ludmilae sp. n. and C. ameliae sp. n. these setae are simple, in C. flava all these setae are bifurcate ( Fig. 14 View FIGURES 10 – 15 ).
Based on the presence of arched row of setae on tibia, Lugo-Ortiz and McCafferty (1996b) regarded Crassabwa to be related with Cloeodes Traver 1938 and placed them to the « Cloeodes complex», together with Dabulamanzia Lugo-Ortiz & McCafferty 1996a ( Lugo-Ortiz & McCafferty 1998b). Actually, such setae form a row on proximal part of tibia in various taxa of Baetidae , including well-known Cloeonini ( Kluge & Novikova 1992: Figs 1, 2 View FIGURES 1 – 4 ); this is considered a plesiomorphy by Salles et al. (2016).
(15) Apical setal patch on larval tibia. Small, arched setae, which are dispersed over larval leg, are especially dense on apex of inner (ventral) side of tibia, forming here a setal patch. Size of this setal patch varies individually; it is either limited by apical portion of tibia ( Figs. 16–17 View FIGURES 16 – 19 ), or is stretched along tibia.
Besides Crassabwa View in CoL , such setal patch is present also in Susua View in CoL and Dabulamanzia View in CoL ; more compact and dense setal patch on the same place is present in Centroptiloides Lestage 1918 View in CoL and Dicentroptilum Wuillot & Gillies 1994 View in CoL ; setae of the same type are constantly present on the same place in some other taxa.
(16) Stout setae on larval tibia. Outer side of tibia and tarsus have no stout setae; outer-apical seta of tibia is absent as well ( Figs 10–12 View FIGURES 10 – 15 , 64 View FIGURES 60 – 65 , 67 View FIGURES 66 – 75 , 122 View FIGURES 119 – 124 , 125 View FIGURES 125 – 132 ).
(17) Imaginal and subimaginal tibia and tarsus. In all 3 species described below, in imago and subimago of both sexes tibia of each leg with more or less prominent inner-apical projection ( Fig. 103 View FIGURES 96 – 103 ). Tarsus moderately long ( Figs 31, 32, 34 View FIGURES 31 – 36 , 97, 101, 102 View FIGURES 96 – 103 ). Middle and hind legs of both sexes and on fore leg of female have 2 apical spines on 2nd and 3rd primary tarsomeres; 3rd apical spine, located on 4th primary tarsomere (penultimate one) either present, or absent on individual legs ( Figs 103 View FIGURES 96 – 103 , 122 View FIGURES 119 – 124 ).
(18) Microlepides on subimaginal tarsi. In all 3 species described below, all tarsal segments of all legs of both sexes are covered by pointed microlepides. The same in most Baetidae other than Baetungulata.
(19) Larval claw. Claw with two regular rows of denticles, among which two most distal denticles of each row are enlarged and shifted toward sides of claw. These two rows of denticles (anterior and posterior rows) are located symmetrically on inner side of the claw; in C. ludmilae sp.n. and C. ameliae sp. n. they are also symmetric regarding size of their denticles ( Figs 66 View FIGURES 66 – 75 , 95 View FIGURES 84 – 95 , 126 View FIGURES 125 – 132 ), but in C. flava two distal denticles of the anterior row are large, while two distal denticles of the posterior row are twice smaller ( Figs 15 View FIGURES 10 – 15 , 18–19 View FIGURES 16 – 19 ). On individual claws instead of two large distal denticles, there is larger number of thinner denticles.
Besides Crassabwa , two distal denticles in each of two rows are enlarged and shifted laterally in Susua , that is a possible synapomorphy of Crassabwa and Susua . However, two enlarged distal denticles in each of two rows are found in some other taxa, e.g. in selected species of Cheleocloeon ( Kluge 2016: Figs 23 View FIGURES 23 – 30 , 37 View FIGURES 37 – 39 ) and in undescribed species of Indocloeon .
Lugo-Ortiz & McCafferty (1996b) overlooked posterior row of denticles on the claw of C. flava and wrongly described it as having one row of denticles. Probably because of this error, they wrongly compared Crassabwa with Dabulamanzia , which has one row of denticles on larval claws ( McCafferty 1999), but did not compare Crassabwa with Susua .
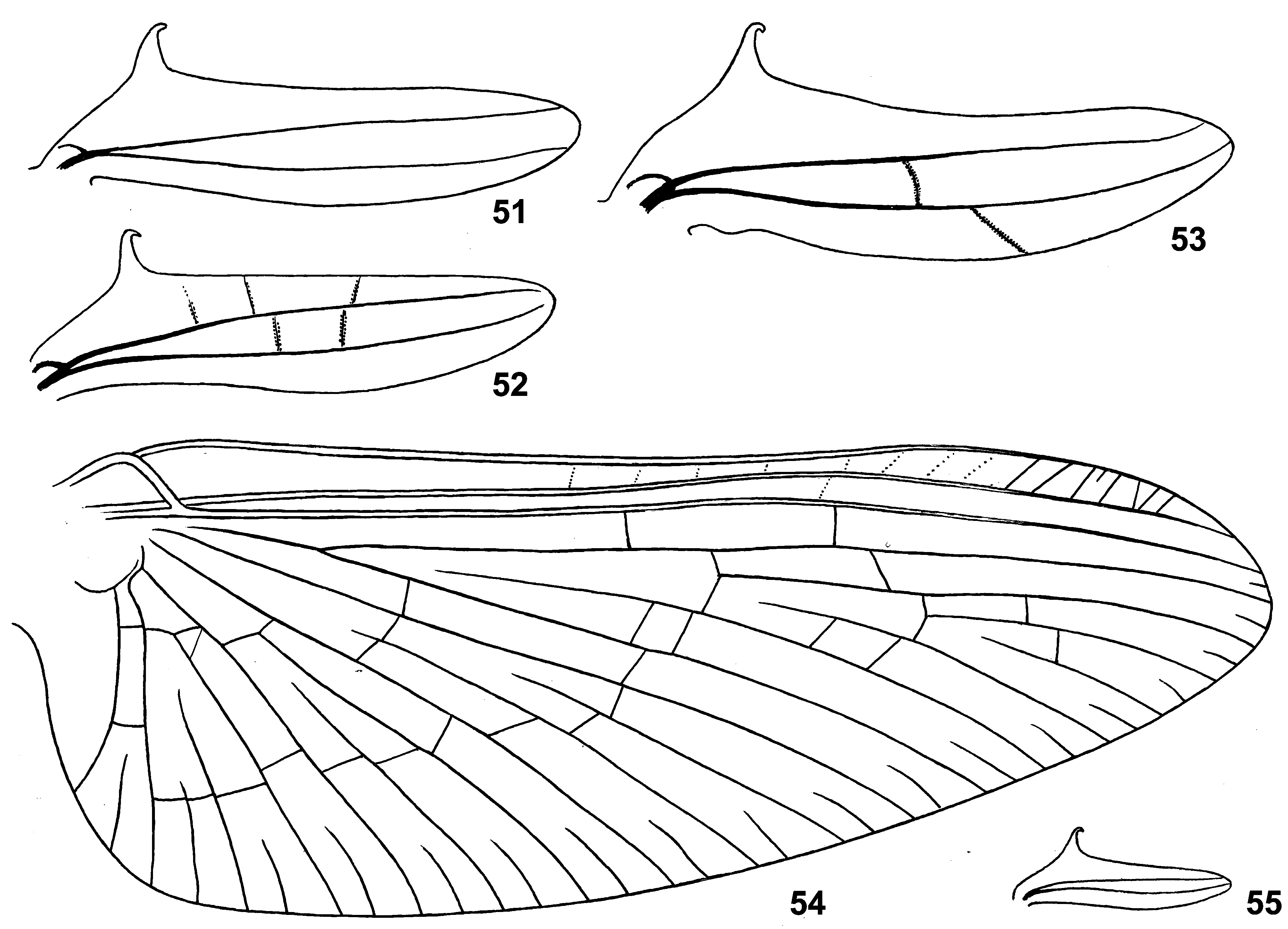
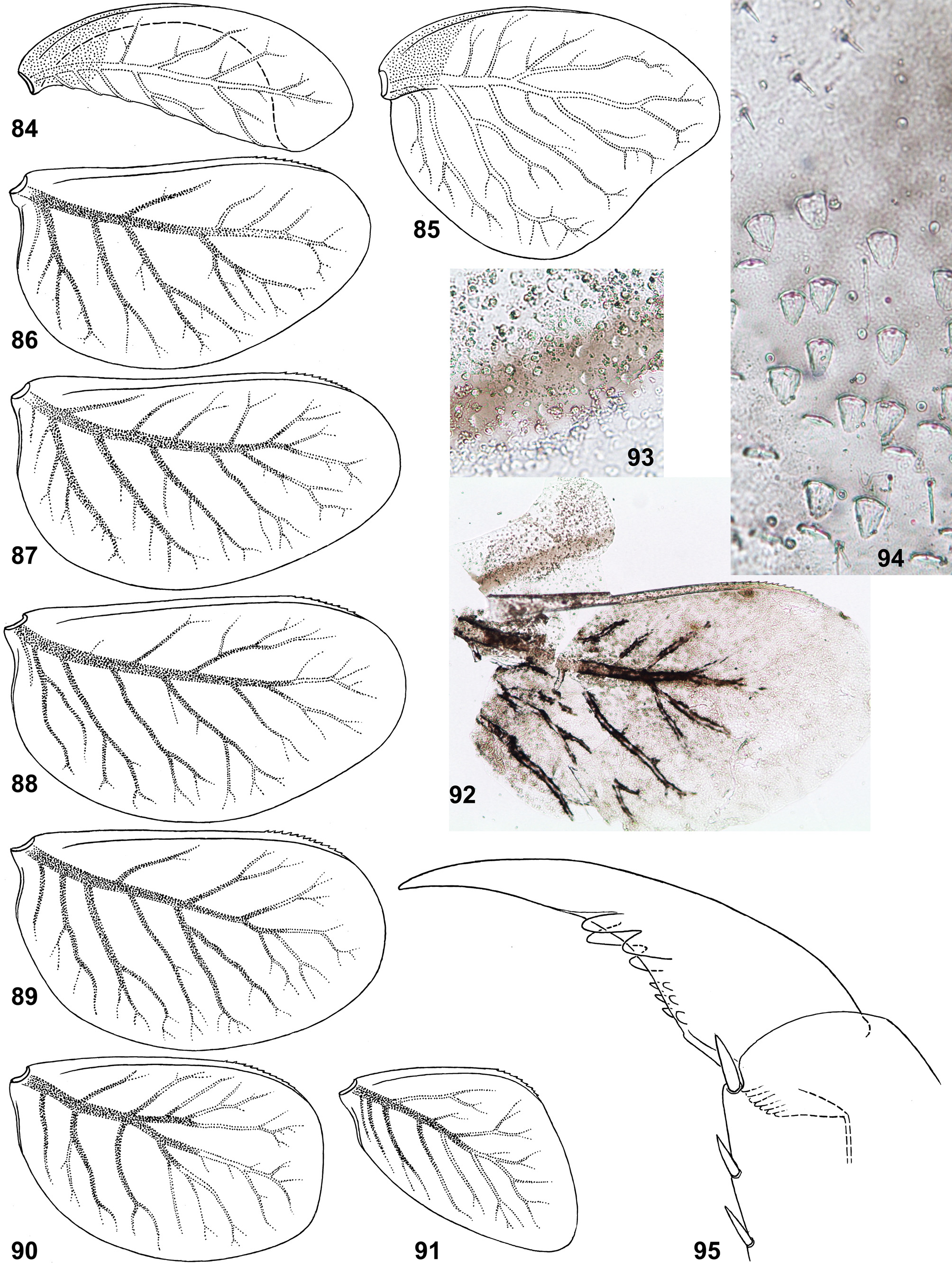
(20) Fore wing. Fore wing is widest near base, gradually narrowing toward apex ( Fig. 54 View FIGURES 51 – 55 ; Crass 1947: 20d). The same in various relatively large Baetidae , in contrast to smaller Baetidae (including Susua ) whose wings are more oval and widest near middle.
Pterostigma of fore wing has approximately 5–20 oblique veins, which can be complete and incomplete, simple and branches, well-expressed and vestigial; several weak cross veins can be present proximad of pterostigma; one marginal intercalary of this or that length either present in each space, or absent in one or two most posterior spaces.
(21) Hind wing. Hind wing present in both sexes, of « Centroptilum -type»: narrow, with fore margin nearly straight and hind margin slightly convex, with 2 longitudinal veins, with hooked costal projection; in contrast to other taxa with the « Centroptilum -type» hind wing, in Crassabwa costal projection is especially long and directed perpendicular to the wing ( Figs 51–53, 55 View FIGURES 51 – 55 ; Crass 1947: Fig. 20f View FIGURES 20 – 22 ). The same in the species originally described as Cloeon vitreum Navás 1930 (see below).
(22) Denticles on larval abdomen. In all 3 species, posterior margins of abdominal terga II–X with regular row of large, narrow, pointed denticles, which can alternate with smaller denticles ( Fig. 20 View FIGURES 20 – 22 ); posterior margin of abdominal tergum I either with the same denticles, or with smaller denticles, or lack denticles (individual variability); at middle of posterior margin of tergum IX denticles either slightly diminished ( Fig. 65 View FIGURES 60 – 65 ), or indistinguishable from others, never interrupted. Anterior abdominal sterna without denticles, posterior sterna (sterna VI–IX of C. flava , or sterna V–IX of C. ludmilae sp. n. and C. ameliae sp. n.) with regular row of pointed denticles smaller than denticles on terga; sternum IX of male with pointed denticles between protogonostyli and laterad of them ( Fig. 104 View FIGURES 104 – 107 ). Each paraproct with 6–10 large pointed denticles ( Fig. 68 View FIGURES 66 – 75 ; Lugo-Ortiz & McCafferty 1996b: Fig. 12 View FIGURES 10 – 15 ).
(23) Scales on larval abdomen. Abdominal terga with numerous scales; in C. ludmilae sp. n. and C. ameliae sp. n. all scales are short and blunt ( Fig. 94 View FIGURES 84 – 95 ); in C. flava scales vary from short and blunt to long and pointed ( Figs 20–21 View FIGURES 20 – 22 ), that is similar to scales of Susua . Abdominal sterna with short scales only ( Fig. 22 View FIGURES 20 – 22 ).
(24) Setae on larval sterna. In all three species abdominal sterna bear only sparse short setae; in C. ludmilae sp. n. and C. ameliae sp. n. all setae are simple (not bifurcate) and do not form rows; in C. flava several setae located near lateral rugose convexity, are bifurcate and sometimes form irregular row, which is either nearly longitudinal (stretching laterad of the rugose convexity), or nearly transverse (stretching posteriad of the rugose convexity), or arched ( Fig. 22 View FIGURES 20 – 22 ).
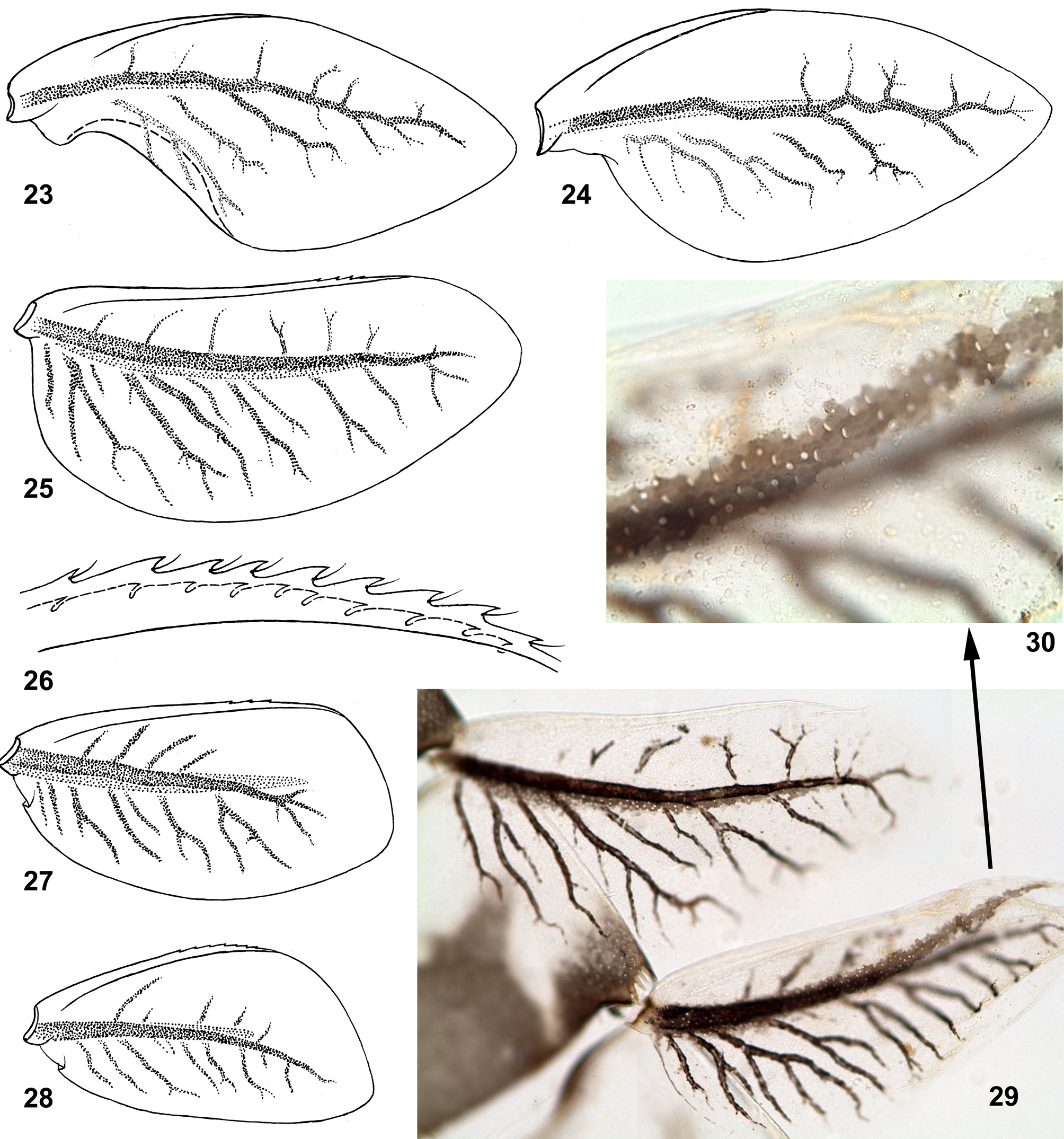
(25) Structure of tergalii. Tergalius I with anal margin not bordered by rib and convex in proximal part ( Figs 24 View FIGURES 23 – 30 , 85 View FIGURES 84 – 95 ); in larva of last instar this convex proximal portion is overturned and bent ventrally ( Figs 23 View FIGURES 23 – 30 , 84 View FIGURES 84 – 95 ). In previous larval instars tergalius I is not folded in such a manner, being spread similar to Figs 24 View FIGURES 23 – 30 and 85 View FIGURES 84 – 95 . Among examined last instar larvae of three species, only in few individuals selected tergalius I (either left or right one), was not folded, but spread as in younger larvae.
Other tergalii II–VII have no overturned anal lobe; their anal margin either has no anal rib ( Fig. 25 View FIGURES 23 – 30 ), or has very short anal rib ( Figs 86–91 View FIGURES 84 – 95 ), that varies individually and among tergalii of one individual ( Fig. 29 View FIGURES 23 – 30 ). Costal margin is armed by costal rib, which in its distal part bears small seta-bearing denticles ( Fig. 26 View FIGURES 23 – 30 ).
Superficially, last instar larval tergalii of Crassabwa resemble that of Susua , Dabulamanzia and Cheleocloeon , whose tergalii I are petiolate and sharply different from others; in contrast to these taxa, in Crassabwa tergalii I are not really petiolate, but get this shape due to folding. Among Baetidae , besides Crassabwa , tergalii with overturned anal lobe are found only in Cloeonini and Callibaetini; in contrast to Crassabwa , anal lobe of Cloeonini is bent not ventrally, but dorsally and can be present on tergalii of several pairs; in Callibaetini the anal lobe bent ventrally is present on tergalii of several or all pairs.
(26) Pigmentation of tergalii. Dorsal cuticle of each tergalius I–VII can have a brown stripe running just above main trachea; in C. flava this stripe is wider than trachea ( Figs 29–30 View FIGURES 23 – 30 ); in C. ludmilae sp. n. this stripe is not wider than trachea, so that is invisible on background of trachea ( Figs 92–93 View FIGURES 84 – 95 ); in some individuals of C. ludmilae sp. n. and C. ameliae sp. n. this stripe is completely absent.
(27) Mobility of tergalii. Tergalii make rhythmical respiratory movements with little amplitude. In contrast to other mayflies able to such movements, larvae of Crassabwa are unable to live in stagnant water, so it is rather difficult to transport living larvae from place of collecting to a cage placed on current, and impossible to keep them in container for rearing. Crass (1947) also noted that «The nymphs have proved very intolerant of captivity».
(28) Larval abdominal tergum X. Anterior margin of abdominal tergum X forms a pair of prominent projections ( Fig. 65 View FIGURES 60 – 65 ), which serve as places of attachment for various longitudinal dorsal muscles running from anterior margin of tergum IX and from previous abdominal terga. In other taxa, including Susua , anterior margin of this tergum is either nearly straight, or forms a pair of shallow convexities.
(29) Larval caudalii. Caudalii of the «Siphlonuroid-type»: cerci and paracercus have equal length and bear long, dense primary swimming setae; larvae of Crassabwa are good swimmers. Posterior margin of each caudalius segment bears denticles, which are enlarged on lateral sides of cerci and on dorsal side of paracercus, being somewhat larger on each 2nd segment ( Fig. 63 View FIGURES 60 – 65 ) (in contrast to Susua , whose denticles are much smaller). In distal half of cercus outer side with more or less developed secondary swimming setae, not forming regular row ( Fig. 63 View FIGURES 60 – 65 ).
(30) Pose of developing gonostyli. Before molt from larva to subimago, subimaginal gonostyli are bent under larval cuticle in « Cloeon - type » pose, i.e. with 2nd segments directed laterally ( Fig. 104 View FIGURES 104 – 107 ); this is known for all 3 species described below (the same in most other Protopatellata and Anteropatellata-non-Baetovectata).
(31) Styliger of male imago. At least in C. flava and C. ludmilae sp. n. apical margin of each unistyliger bears a median projection, which represents continuation of dorsal wall of unistyliger and is projected in medio-ventral direction ( Figs 37–39 View FIGURES 37 – 39 , 41 View FIGURES 40 – 44 , 106 View FIGURES 104 – 107 ).
(32) Penis of male imago. At least in C. flava and C. ludmilae sp. n. inner margin of penial bridge forms a pair of internal projections directed cranially and located dorsad of gonovectes ( Figs 40 View FIGURES 40 – 44 , 107 View FIGURES 104 – 107 ). The same in Susua .
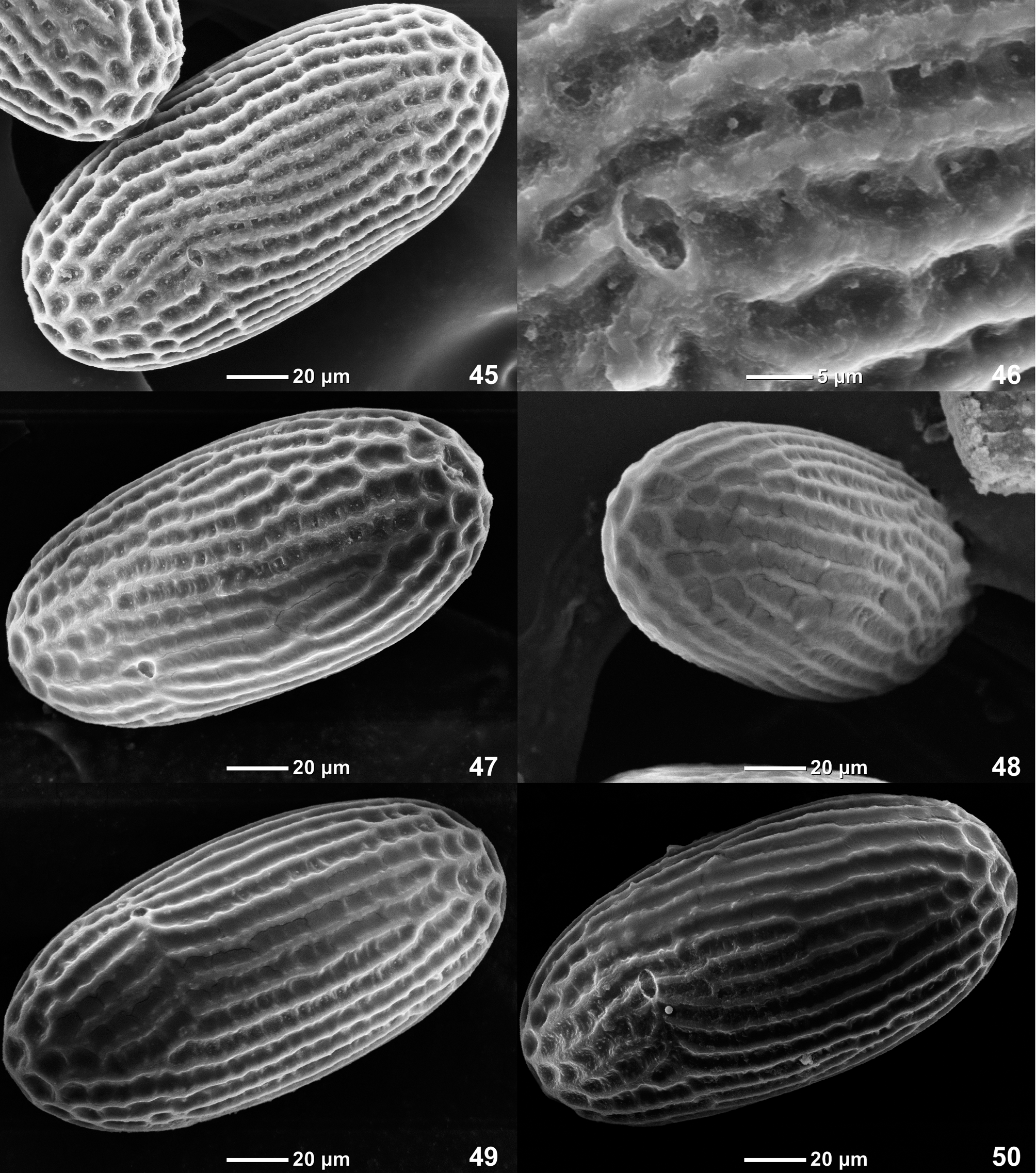
(33) Eggs. At least in C. flava and C. ludmilae sp. n. eggs are elongate, chorion with net-like relief, whose longitudinal ridges are more wide and convex than transverse ridges, so that in middle part of egg this relief looks as longitudinal striation ( Figs 45–50 View FIGURES 45 – 50 , 108–113 View FIGURES 108 – 113 ). This relief allows to distinguish Crassabwa from many other Baetidae , whose chorion has even net-like relief with isodiametric cells separated by equally developed longitudinal and transverse ridges. Susua niandanense also has irregular longitudinal ridges in middle part of egg (paper in preparing); possibly, this is a synapomorphy of Crassabwa and Susua .
Distribution. Afrotropical Region.
Discussion about species composition of Crassabwa . Lugo-Ortiz & McCafferty (1996b) established the genus Crassabwa for 4 species, which were originally described as Centroptilum flavum Crass 1947 (the type species of Crassabwa ), Centroptilum loweae Kimmins 1949 , Centroptilum badium Kopelke 1980 and Cloeon vitreum Navás 1930 .
Centroptilum loweae was originally described by Kimmins (1949) as male imagoes, male subimagoes and female subimagoes collected near Lake Nyasa, with a male imago designated as the holotype. Male imago of C. loweae belongs to Cheleocloeon Wuillot & Gillies 1993a View in CoL , about which testifies structure of its gonovectes (which Kimmins erroneously termed «titillators») with hooked apices «a pair of sinuous titillators, whose apices are bent at right-angles and overlapping» ( Kimmins 1949: 829, Fig. 4 View FIGURES 1 – 4 ); this genital structure allows to distinguish Cheleocloeon View in CoL from all other taxa ( Kluge 2016). Kimmins noted similarity of C. loweae with Centroptilum excisum Barnard 1932 (which is recently placed to Cheleocloeon View in CoL ) and stated that C. loweae differs from C. excisum by presence of hind wings in female. At the same time Kimmins (1949) did not prove that female subimagoes reported in his paper as C. loweae , are really conspecific with the male imaginal holotype of C. loweae . Actually females of all Cheleocloeon View in CoL have no hind wings ( Kluge 2016). According to our data (Kluge, unpublished), two species of Dabulamanzia View in CoL occur in tributaries of Lake Nyasa; their female subimagoes and imagoes are similar to that of Cheleocloeon View in CoL , but differ in having hind wings. So it is quite probable that female paratypes of Centroptilum loweae belong to some of these species of Dabulamanzia View in CoL . In another paper, Kimmins (1960) described male and female imagoes and subimagoes attributed by him to Centroptilum loweae , collected near Lake Victoria (in Uganda and Tanzania), and noted variability of this species. Possibly, here also various species were reported under the name « Centroptilum loweae ». Gillies (1990) placed the species loweae [ Centroptilum View in CoL ] to the group tarsale of the genus Afroptilum View in CoL , and Lugo-Ortiz & McCafferty (1996b) placed it to Crassabwa View in CoL . According to the original designation of male imago from Lake Nyasa as the holotype (originally termed « type »), the species loweae [ Centroptilum View in CoL ] undoubtedly belongs to Cheleocloeon View in CoL , and can be treated as Cheleocloeon loweae (Kimmins 1 949) comb. n. Not far from the Lake Nyasa, in the same mountain system (in the source of Great Ruaha River near Mfumbi and in a tributary of Little Ruaha River near Iringa), the first author reared imagoes from larvae of Cheleocloeon excisum ( Barnard 1932) View in CoL . All characters of this species agree with the description of the holotype of C. loweae , so the following synonymy is proposed here: Ch. excisum View in CoL = Ch. loweae syn. n.
Centroptilum badium Kopelke 1980 was originally described as male and female imagoes from D.R. Congo ( Zaire). Gillies (1990) placed it to the group tarsale of the genus Afroptilum View in CoL , and Lugo-Ortiz & McCafferty (1996b) placed it to Crassabwa View in CoL . One of us (N. Kluge) has reared material of this species from Uganda; it belongs to a new genus, which will be described in a separate publication.
Cloeon vitreum Navás 1930 was described as a single female imago from Congo, and its systematic position remains doubtful (see below).
Thus, only Crassabwa flava ( Crass 1947) View in CoL was reliably attributed to the genus Crassabwa View in CoL . This species is redescribed below based on reared material. Two other species, Crassabwa ludmilae sp. n. and C. ameliae sp. n. are described below.
Discussion about diagnosis of Crassabwa . The original diagnosis of Crassabwa included characters of larva and imagoes ( Lugo-Ortiz & McCafferty 1996b). Larval claw of Crassabwa was characterized as having «two enlarged subapical denticles and four to six small basal denticles» ( Lugo-Ortiz & McCafferty 1996b: 236), that was regarded to be different from the « Centroptiloides complex», whose larval claw was said to «possess 2 subparallel rows of denticles» ( Lugo-Ortiz & McCafferty 1998a: 2). Actually, larval claws were wrongly described for a number of baetid species, including Crassabwa flava , whose claws have not one, but two rows of denticles, with two enlarged distal denticles in each row ( Figs 15 View FIGURES 10 – 15 , 18–19 View FIGURES 16 – 19 ) [see character (19)]. If correct this character, it appears that Lugo-Ortiz & McCafferty (1996b) did not report any character of Crassabwa different from Susua , which was placed by them to the « Centroptiloides complex» ( Lugo-Ortiz & McCafferty 1998a). Actually, Crassabwa and Susua are closely related [see characters (12), (19)]. Larva of Crassabwa differs from larva of Susua mainly by structure of 1 st tergalii , which in Crassabwa have a proximal-anal flap overturned ventrally in the last larval instar ( Figs 23 View FIGURES 23 – 30 , 84 View FIGURES 84 – 95 ). This character of Crassabwa was not reported neither by Crass (1947), nor by Lugo-Ortiz & McCafferty (1996b), who described and figured only tergalii («gills») of 4th pair.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Crassabwa Lugo-Ortiz & McCafferty 1996
| Kluge, Nikita J., Gattolliat, Jean-Luc & Salles, Frederico F. 2017 |
Crassabwa
| Lugo-Ortiz 1996: 236 |