Gnatholepis thompsoni Jordan , 1904
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3529.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A16A2C8E-8074-4B5C-B097-4C365DBB77C2 |
|
persistent identifier |
https://treatment.plazi.org/id/7B14879F-FF85-E234-FF40-FE16FD7303F2 |
|
treatment provided by |
Felipe |
|
scientific name |
Gnatholepis thompsoni Jordan , 1904 |
| status |
|
Gnatholepis thompsoni Jordan, 1904 View in CoL
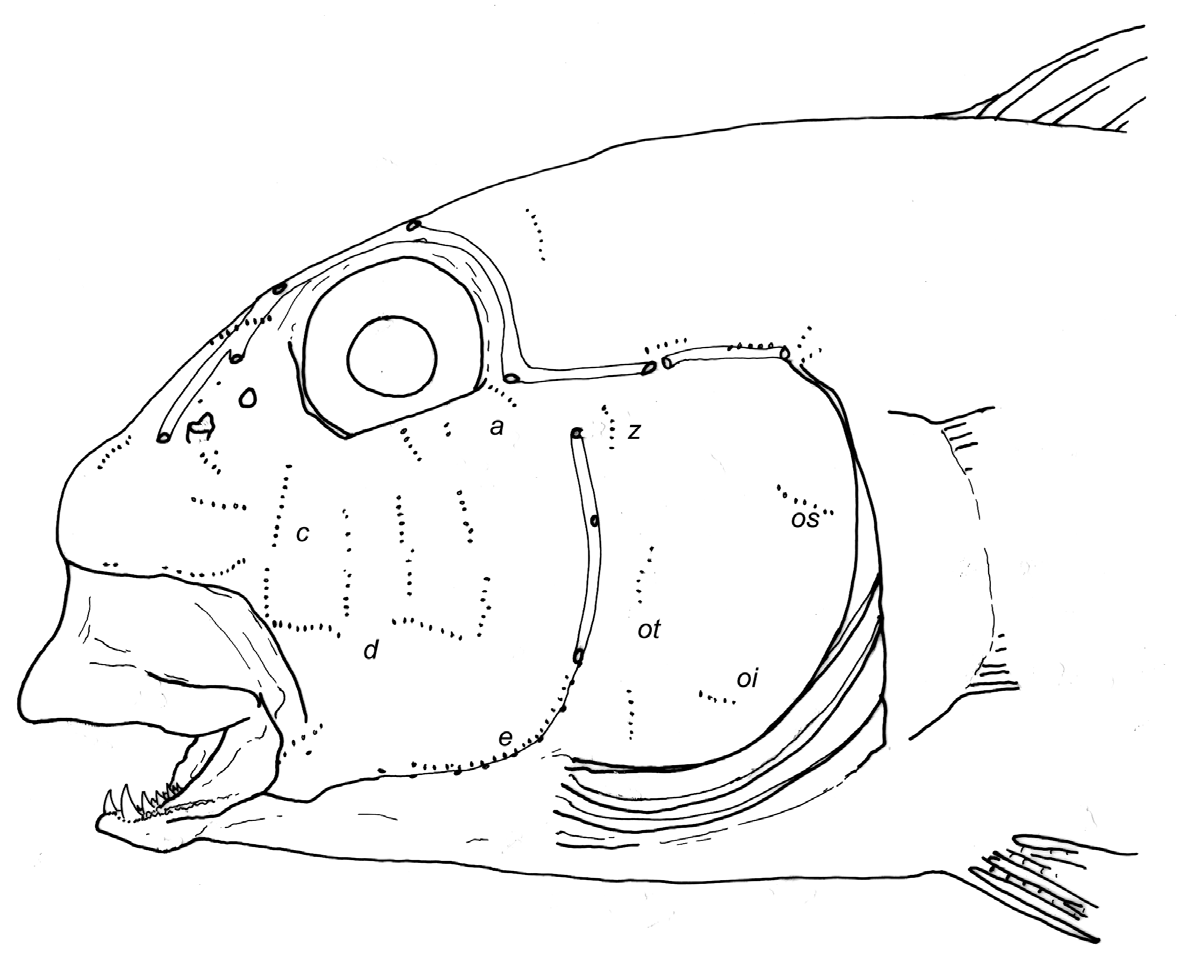
( Figs 4E–F View FIGURE 4 , 12F View FIGURE 12 , 25–26 View FIGURE 25 View FIGURE 26 ; Tables 5–8, 14)
Gnatholepis thompsoni Jordan, 1904: 541 View in CoL , pl. 1, fig. 2 (Bush Key, Tortugas Archipelago, Florida).— Böhlke 1953: 113; Randall 1968: 248–249 ( Bermuda; Caribbean Sea); Lubbock 1980: 296 ( Ascension Island); Robins and Ray 1986: 246 ( Bahamas); Edwards and Glass 1987: 659 (St Helena); Miller 1990: 931; Smith-Vaniz et al. 1999: 316 ( Bermuda); Schwartz 1999: 284 (North Carolina); Brito and Miller 2001: 255 View Cited Treatment , Fig. 2A View FIGURE 2 (Cape Verdes); Gasparini and Floeter 2001: 1646 (Trindade Island, Brazil); Rocha and Rosa 2001: 992 (Maranhão, Brazil); Randall and Greenfield 2001: 1; Thacker and Cole 2002: 840; Araújo and Freitas 2002: 2 (Madeira Island); Collette et al. 2003: 122 ( Navassa Island); Murdy and Hoese 2003: 1795; Smith et al. 2003: 62 ( Belize); Ross and Rhode 2004: 309, plate IIId (Onslow Bay, North Carolina); Nelson et al. 2004: 171; Rocha et al. 2005: 1–6.
Gnatholepis sp. —Lubbock in Miller 1990: 931 ( Ghana).
Bathygobius soporator View in CoL — Debelius 1997: 244 ( Cape Verde Islands) [misidentification].
Gnatholepis scapulostigma View in CoL — Brito and Miller 2001: 258 ( Cape Verde Islands).
Diagnosis. A moderately large Gnatholepis (up to 58 mm SL) with nape midline scales always cycloid and most of predorsal scales cycloid; body pale with 6–8 rows of staggered dark brown spots, mid-lateral spots may be largest; transverse black line on the upper part of the eye joining somewhat oblique to curved black line or bar crossing cheek and ending well behind end of jaw; third to fourth first dorsal fin spines longest, fin with square to rectangular appearance when extended; second dorsal and anal fin rays usually I,11; pectoral rays 16–18, usually 17; lateral scales 26–29, usually 27; 9–11 predorsal scales (usually 10), all cycloid.
Material examined. BELIZE: USNM 276135 About USNM , 12 About USNM (10–41.5), E side Carrie Bow Cay, D.J. Johnson and P. Keener, 12 November 1984 ; USNM 276131 About USNM , 4 About USNM (18.5–30.5), S end Carrie Bow Cay, D.J. Johnson and P. Keener, 5 November 1984 ; USNM 346472 About USNM , 2 About USNM (27–33.5), Carrie Bow Cay, D. Smith, C. Thacker, 16 September 1997 . NICARAGUA: USNM 362225 About USNM , 3 About USNM (24.5–30), Narrow Cay, Serrana Bank, Oregon Cruise 78, 20 May 1962 ; USNM 320763 About USNM , 2 About USNM (27–36.5), off Corn Island , Caribbean, Oregon Cruise 78, 2 June 1962 . BAHAMAS: USNM 198783 About USNM , 1 About USNM (33.5), S end of beach near canal inlet, Lyford Cay, Clifton Bay , W.L. Schmitt, 16 August 1961 . CUBA: USNM 82520 About USNM , 6 About USNM (29–44), San Antonio, Tomas Barreras Expedition, Henderson and Bartsch, 4 June 1914 . JAMAICA: LACM 5974 About LACM , 12 About LACM (34.5–58), Pedro Cays, D.K. Caldwell, 21 April 1959 . LESSER ANTILLES: USNM 264886 About USNM , 1 About USNM (58), E side Cocoa Point, Barbuda, 25 April 1959 . VENZUELA: USNM 179253 About USNM , 1 About USNM (41.5), Sarqui Islands, Los Roques Islands , P. Bottome and Wallis, 4 July 1958 ; USNM 179252 About USNM , 11 About USNM (31.5–51), Yonqui, Los Roques Islands , P. Bottome and Wallis, 5 July 1958 ; USNM 179254 About USNM , 3 About USNM (14–37), Sarky Island , Los Roques, P. Bottome and Wallis, 27 June 1958 . TRINIDAD AND TOBAGO: USNM 317097 About USNM , 10 About USNM (11–41), leeward side Little Tobago Island, J . T. Williams and party, 6 September 1990 ; USNM 317098 About USNM , 8 About USNM (12–34), Man-of-War Bay, Booby Island , Tobago, J . T. Williams and party, 14 September 1990 . BRAZIL: USNM 357711 About USNM , 3 About USNM (34.5–40.5), Atol das Rocas, Rio Grande do Norte, N.A. Menezes, February 1972 ; USNM 368819 About USNM , 1 About USNM (28), Bay of Turiacu , 9 March 1963 . ASCENSION ISLAND: USNM 368871 About USNM , 1 About USNM (30), rocky point at N edge of English Bay , R. Manning, P. Kashulines, 20 May 1971 ; USNM 218840 About USNM , 1 About USNM (44), McArthur Point, M.L. Jones and party, 11 July 1976 . ST HELENA: USNM 267896 About USNM , 6 About USNM (25–36), Lemon Valley Bay , A. Edwards and C. Glass, 28 June 1983 .
Other material; no data taken. ASCENSION ISLAND: USNM 368859, 2.
Description. Based on 61 specimens, 21.5–58.0 mm SL. An asterisk indicates the counts of the holotype of Gnatholepis thompsoni (data provided by Dave Catania of CAS).
7 (usually 7/6); lateral scale count 26–29 (mean 27.0*); TRB 9 *–12 (mean 10.4); predorsal scales 9–11.5 (mean 9.8, 10 in holotype); circumpeduncular scales 12–14 (mean 12.1). Gill rakers on outer face of first arch 1–2 + 3–5 (in 8, usually 1+4) .
Body compressed, width at anus 9.8–16.0% (mean 13.2%) of SL. Body rather slender, body depth at anus 17.7–25.7% (mean 21.5%) of SL, body depth at first dorsal fin origin 19.3–25.3% (mean 22.4%) of SL. Head compressed, slightly broader ventrally, deeper than wide, HL 25.7–30.0% (mean 28.0%) of SL; head depth at posterior preopercular margin 58.5–76.6% (mean 67.5%) of HL; head width at posterior preopercular margin 57.3–77.2% (mean 67.7%) of HL; head profile bluntly pointed to rounded; nape profile slightly curved to flat. Mouth subterminal to nearly terminal, slightly oblique; jaws generally reaching to below anterior half of eye; upper jaw length 28.1–39.0% (mean 34.4%) of HL. Upper lip smooth, narrower than lower, lower lip with papillose ridge close to teeth, with twist or fold posteriorly, forming triangular flap, lip narrowly interrupted at chin. Eye moderate to relatively small, dorsolateral, 23.0–35.5% (mean 27.0%) of HL; preorbital width 13.6–24.8% (mean 19.4%) of HL. Snout bluntly pointed to rounded, 25.7–38.2% (mean 30.9%) of HL; posterior naris rounded, close to anterior margin at middle of eye; anterior naris in short tube, higher on posterior margin of eye, about level with middle of eye or somewhat ventral to it. Interorbital narrow, 4.9–11.0% (mean 7.9%) of HL. Caudal peduncle compressed, length 14.3–18.6% (mean 16.5%) of SL; caudal peduncle depth 10.2–13.8% (mean 11.6%) of SL.
First dorsal fin rounded to roughly triangular, nearly square in large adults, with no spines elongate; second to fourth spine longest; when adpressed, spine tips reaching to first to third element of second dorsal fin. Second dorsal spine length 14.9–20.3% (mean 17.2%) of SL; third dorsal spine length 15.8–21.6% (mean 18.0%) of SL. Second dorsal fin as tall or nearly as tall as first dorsal fin, posteriormost rays usually longer than anterior, fin pointed to slightly rounded posteriorly. Anal fin lower than second dorsal fin, anteriormost rays shorter than posterior few rays; fin usually pointed posteriorly. Second dorsal and anal fin rays, when adpressed, just reaching caudal fin rays. Pectoral fin oval to somewhat pointed, central rays longest, 22.5–29.8% (mean 26.0%) of SL; fin reaching back to above first few anal fin elements. Pelvic fins fused, frenum with distinctive finely fimbriate margin, fins round to somewhat oval, reaching to first one or two anal fin elements, 23.3–31.0% (mean 27.2%) in SL. Caudal fin moderate, oval, rounded to slightly pointed, 26.6–37.4% (mean 31.8%) of SL.
Gill opening restricted, extending anteriorly to lower edge of pectoral base or to just under opercle. One or two slender, fleshy gill rakers on outer face of first arch, close to angle of arch, remaining few rakers shorter, pointed; outer rakers on second gill arch consisting of two series of papillae, one low and one (mostly anterior) of slightly pointed papillae; outer rakers on remaining arches low, stubby, broad-based. Inner face of upper limb of first gill arch, and to lesser extent, upper limbs of other arches, covered with low dense fleshy papillae, usually forming clumps or groups; dorsal portion of first arch may have short fleshy protuberances ending in one or several papillae; inner face of second arch smooth, without papillae but with about 12 short fleshy rakers with rounded, finely papillose tips. About one quarter or less of first gill arch bound by membrane to inner face of opercle. Tongue short, tip bilobed to concave.
Teeth in upper jaw in two to three rows across front and one row at side of jaw, outermost row teeth largest, curved and pointed, largest teeth at front of jaw on either side of symphysis (smaller in females); innermost row teeth very small, sharp and evenly sized. Teeth in lower jaw in two to three rows, arranged similarly to upper jaw but outer row teeth may be smaller (in females); posteriormost one or two outer row teeth enlarged and somewhat recurved in males.
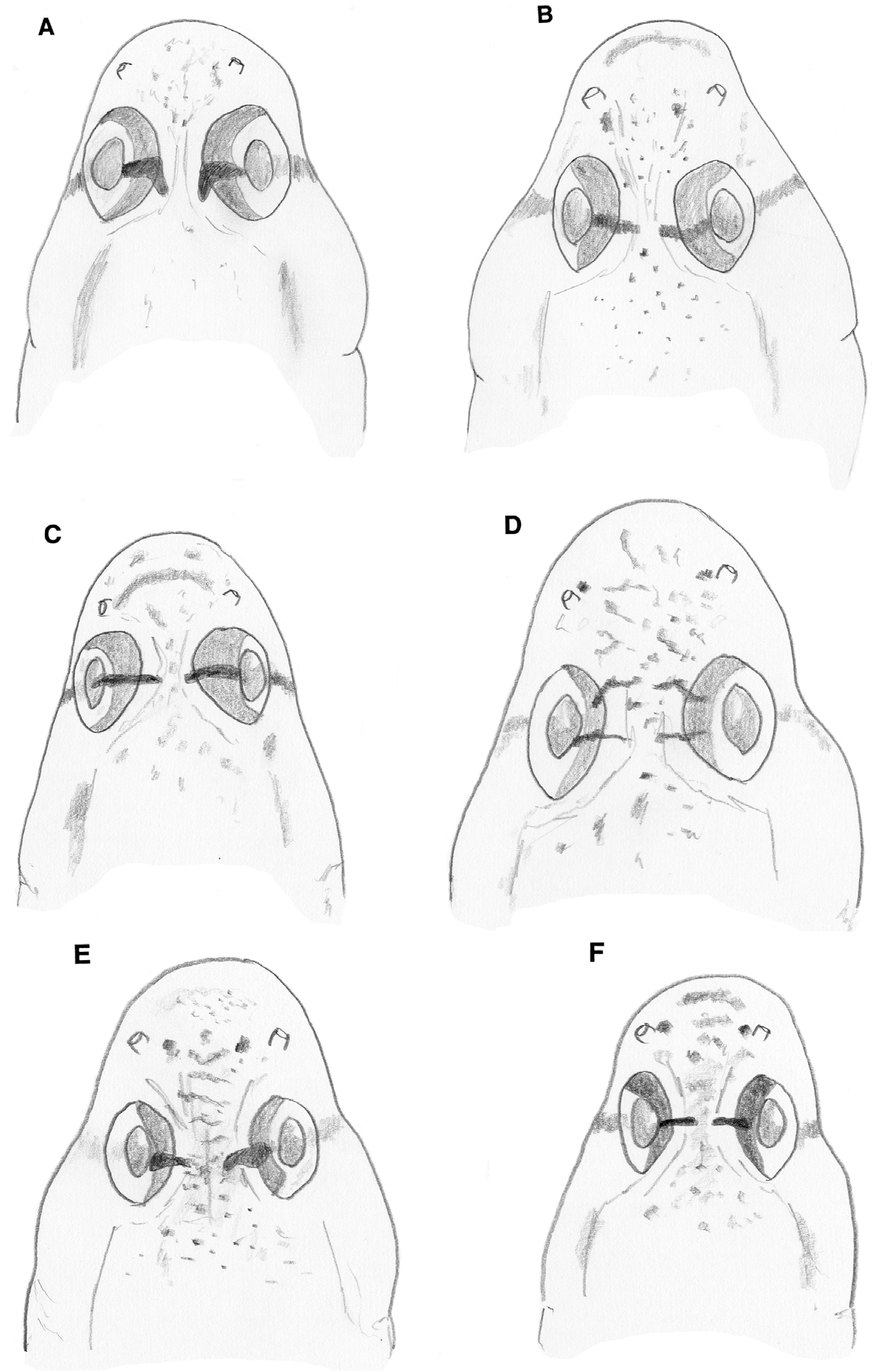
Predorsal scales cycloid, ctenoid body scales reaching forward to rear corner of opercle to just over opercle, in two specimens (from Brazil and Ascension), ctenoid scales reaching forward to over preopercle; nape midline scales always cycloid ( Fig. 4E–F View FIGURE 4 ). Opercle covered with cycloid scales, a few ctenoid scales on upper part may be present ( Jamaica specimens with mostly ctenoid scales). Preopercular scales cycloid, occasionally extending anterior to vertical dark cheek bar below eye. Breast with cycloid scales reaching up to below mid-opercle or to below rear half of opercle. Pectoral fin base covered with cycloid scales; occasionally with few ctenoid scales in centre. Belly scales along midline usually cycloid; ctenoid scales may be present posteriorly .
Head pores as in G. anjerensis ( Fig. 1 View FIGURE 1 ).
Sensory papillae as in G. anjerensis .
Coloration of fresh material. Published photographs of living or freshly dead fish can be seen in Debelius (1997: 244, as Bathygobius soporator ), Collette et al. (2003: 123, fig. 143) and Ross and Rohde (2004). Living and freshly dead fish are very similar in colour to the spotted form of G. cauerensis .
Description from colour photographs of living fish by Rob Myers ( Figs 25–26 View FIGURE 25 View FIGURE 26 ). Head and body dull to pearly white with six to eight rows of many small brown to red-brown round to vertically oval spots running length of body, mid-lateral spots may be partly slightly larger and red-brown (may partly coalesce forming lines); rows of spots underlain by five to six vertically oval, indistinct pale brown to purplish brown bars similar to those in G. anjerensis . Predorsal lightly spotted with pale brown to red-brown. Side of head may be crossed horizontally by red-brown or diffuse brown mottled line extending from just above rictus on to opercle; more often, opercle with (often indistinct) brown to red-brown line from centre of preopercular margin horizontally or obliquely back to opercle rear margin. Narrow to broad black to dark brown cheek bar from ventral edge of eye running vertically down to end on lower preopercular edge. Cheek with diffuse brownish horizontal line crossing vertical cheek bar (diffuse line may continue onto opercle as described above). Dense black to dark brown bar from middle to rear half of iris running over top of eye. Above pectoral fin base, large black U-shaped flat-based blotch surrounding round yellow spot; black blotch may be partly connected to thin red-brown line from behind eye along top of preopercle; indistinguishable in small specimens. Pectoral fin base with horizontal red-brown line crossing middle of fin base.
First dorsal fin translucent pale white, with four to seven rows of many pale purple-brown spots and short streaks (usually forming short lines proximally. Second dorsal fin with similar five to seven rows of brown to purple-brown spots. Anal fin yellowish white to white with blue-white margin; large adult from Florida with short red-brown spot and short streaks along base of fin (difficult to see anal fin in most photos). Caudal fin translucent white, with many irregular rows of small dark brown spots along fin membranes; posterior and ventral margins of fin less pigmented. Pectoral fins transparent with translucent white rays and small fine white speckles basally. Pelvic fins translucent white.
Coloration of preserved material. Based on typically coloured specimens from Jamaica (variation discussed below). Head and body yellowish white to whitish with six or seven rows of many small brown spots running along length of body, joined by variably developed faint brownish lines; rows or lines of spots faintly underlain by five to six vertically oval, brownish bars similar to those in G. anjerensis ; in some specimens, second and third (counting from dorsalmost) and fifth and sixth rows of spots joined by slightly darker background pigment, making these two pairs of rows slightly more conspicuous. Dorsum crossed by about 9–10 usually very faint dusky square saddles
Predorsal, snout and side of head faintly blotched and spotted with pale brown, small spots darkest. Opercle with (often indistinct) pale brown line from centre of preopercular margin obliquely back to upper half of opercle rear margin; line may curve up to rear corner of opercle. Narrow to broad black to light brown cheek bar from ventral edge of eye running vertically to slightly obliquely down to end on lower preopercular edge, but not extending onto branchiostegal membranes. Dense blackish to dark brown line from middle to rear half of iris running over top of eye, often meeting its counterpart in interorbital space ( Fig. 12F View FIGURE 12 ). Upper lip whitish, crossed by short brownish bar (which may be joined by diffuse brown line starting from below anterior naris). Cheek with diffuse brownish horizontal line or blotch crossing vertical cheek bar; brownish line variable in shape and intensity. Above pectoral fin base, large brownish to dark brown, roughly U- or W-shaped flat-based blotch surrounding small very pale brown round spot; brown blotch often partly connected to thin brown line (may be partly broken-up or indistinct) running from behind eye along top of preopercle; pectoral blotch usually paler in small specimens. Pectoral fin base with horizontal brown line crossing just above middle of fin base, line usually extending onto lower part of fin.
First dorsal fin transparent to whitish, with four to six rows of light brown spots and short streaks (often forming lines proximally), rows of spots beginning on first spine (darkest spots); lowermost one or two rows darkest, especially anteriorly; distal part of fin may be plain whitish or translucent. Second dorsal fin with similar five to seven rows of light brown spots or short horizontal streaks. Anal fin plain dusky to brownish, may be darker distally. Caudal fin transparent to translucent brownish, sometimes with pale brown streaks or rows of fine brown spots along fin membranes, especially near fin base and on upper half of fin. Pectoral fins transparent to translucent dusky. Pelvic fins and frenum translucent plain pale brownish, membrane between rays may be darker than frenum.
Variation. Specimens from St Helena and Tobago are similar to the Jamaica fish but the background colour of the head and body is brownish (not whitish or yellowish) and all fins are very heavily spotted with dark brown, and the caudal fin is spotted with black. The markings and spots on the head are curved and almost vermiculate, with the pupil width and the marking above the pectoral fin base is very large and black. Fish from Belize are paler with reduced spotting—about three rows of brown spots dorsally and the rest of the body whitish but with the lateral vertical blotches on the body still visible. The dorsal fin spotting is less defined and the caudal fin is pale with only a few brownish spots basally. Specimens from Atol das Rocas, Brazil, are very pale with no rows of dark spots on the body or fins (which are unmarked); the fish resemble G. cauerensis in colouring. Photos by Rob Myers of fish from San Salvador ( Fig. 26 View FIGURE 26 ) show similar-coloured fish.
Ecology. Randall (1968) reported this species to be common, and found over “sand patches” at depths of 2–160 feet (0.5–48 m). We examined specimens collected from 1–36 m, from sand, coral, rock and rubble substrate (usually sand or rubble).
Sponaugle and Cowen (1994) discussed the early larval life history and recruitment of G. thompsoni , which has a long larval stage and is able to delay settlement. Gnatholepis thompsoni has a larval life of 59–122 (average 81.5) days, and a post-settlement life of about 42 days, with a total lifespan of about 123 days ( Shulman & Bermingham 1995; Thacker 2004a).
Kassi Cole made available to us her unpublished notes on the breeding biology of this species, made during her research project on Coryphopterus at Puerto Rico:
“A nest is formed in a small depression in the sand, under the edge of a coral mound, or under a conch shell (or in my case, under bricks that I set out for Coryphopterus glaucofraenum studies). The eggs are extremely small and are attached to a thin, friable pad of sand which I suspect may be formed by the male with the use of secretions from the accessory gonadal structure associated with the male reproductive system. Both the male and female are associated with the nest, so there is biparental care. When both are at the nest site, often they are positioned with the posterior half of their body under the cover of the overlying structure and the front half out in the open, and often in a 'V' formation with respect to one another, so that their combined visual field is relatively wide.
Whenever I lifted up a brick, the guarding parent(s) would make vigorous side-to-side motions with the caudal end of the body, then shoot away to about a distance of 2 m. At this point, nothing in the nest would be visible to the naked eye. However, when I took a small dive knife and gently inserted it from the side, then moved the flat of the blade back and forth while slowly lifting, the sand pad (or pieces of sand pad) would be lifted up, with the attached eggs. So I concluded that the embryo-guarding parent(s) have a defense behavior that involves covering them with sand before leaving the nest. Presumably, after the disturbance, they return and fan off the sand.
In every instance where I re-checked nests with eggs, the eggs, and guarding behavior, never lasted more than two consecutive days. For example, when a brick was unoccupied one day, then occupied on the next day with guarding fish and eggs, the eggs were present for two consecutive days, then disappeared. In several instances where I removed the sand pad on a second day and placed it into a glass container to bring back to the lab, by the time I got back to the lab the embryos had hatched, apparently due to mechanical stimulation. The newly hatched larvae were extremely tiny. Therefore, I concluded that the embryonic developmental period was extremely short, at around 48 h.” (Cole, in litt., May 2010).
Ross and Rhode (2004) reported two females collected in September from Onslow Bay , North Carolina, one of which had eggs less than 0.1 mm diameter and a gonad index (gonad weight [body weight—gonad weight] –1) x 100) of 0.41; the other had mature eggs of 0.3 mm diameter and gonad index of 3.6 .
Comparisons. This species looks very like G. cauerensis but differs in slightly in predorsal scale count (9–11, modally 10, in G. thompsoni vs 7–12, modally 9, in G. cauerensis ), it has all the predorsal scales cycloid (vs always cycloid in midline, with some ctenoid scales extending over the opercle in G. cauerensis ), having the transverse black line on the upper part of the eye joining the somewhat oblique to curved black line crossing cheek and usually ending well behind end of jaw (vs transverse black bar on cheek mostly vertical to curved, ending past end of jaw) and the dorsal half of the body with three to five rows of small dark spots (dorsal half of body usually with 2–3 rows of short lines in G. cauerensis ; some specimens with rows of spots).
Live specimens of G. cauerensis from South Africa look almost identical in colour pattern to G. thompsoni , as do heavily-spotted forms from dark substrates from Indonesia and Malaysia. The rows of spots along the side of the body in G. thompsoni tend to be fairly even in intensity, while those in G. cauerensis tend to have the dorsalmost four or so rows darker than the remainder. These specimens would be practically impossible to identify from photographs alone, without knowing their geographic origin.
Ascension, Madeira and St Helena, and Sao Tome Island, Cape Verde Islands and the Canary Islands in the eastern Atlantic ( Araujo & Freitas 2002).
Remarks. Rocha et al. (2005) presented a scenario, based on mtDNA, that Gnatholepis invaded the Atlantic about 145,000 years ago from the Indian Ocean, and spread out to the central and eastern Atlantic 100,000 years ago (and are continuing to invade the Canary Islands as sea temperatures rise). They considered G. thompsoni and G. cauerensis (they referred to the latter as G. scapulostigma ) to be sister taxa. Perhaps this is why Thacker (2004a: 579) refers to the Pacific and Caribbean populations of G. thompsoni being separated by the Isthmus of Panama. We know of no Pacific populations of G. thompsoni .
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Gnatholepis thompsoni Jordan , 1904
| Larson, Helen K. & Buckle, Duncan J. 2012 |
Gnatholepis scapulostigma
| Brito, A. & Miller, P. J. 2001: 258 |
Bathygobius soporator
| Debelius, H. 1997: 244 |
Gnatholepis sp.
| Miller, P. J. 1990: 931 |
Gnatholepis thompsoni
| Rocha, L. A. & Robertson, D. R. & Rocha, C. R. & Van Tassell, J. L. & Craig, M. T. & Bowen, B. W. 2005: 1 |
| Ross, S. W. & Rhode, F. C. 2004: 309 |
| Nelson, J. S. & Crossman, E. J. & Espinosa-Perez, H. & Findley, L. T. & Gilbert, C. R. & Lea, R. N. & Williams, J. D. 2004: 171 |
| Collette, B. B. & Williams, J. T. & Thacker, C. E. & Smith, M. L. 2003: 122 |
| Murdy, E. O. & Hoese, D. F. 2003: 1795 |
| Smith, C. L. & Tyler, J. C. & Davis, W. P. & Jones, R. S. & Smith, D. G. & Baldwin, C. C. 2003: 62 |
| Thacker, C. R. & Cole, K. S. 2002: 840 |
| Araujo, R. & Freitas, M. 2002: 2 |
| Brito, A. & Miller, P. J. 2001: 255 |
| Gasparini, J. L. & Floeter, S. R. 2001: 1646 |
| Rocha, L. A. & Rosa, I. L. 2001: 992 |
| Randall, J. E. & Greenfield, D. W. 2001: 1 |
| Smith-Vaniz, W. F. & Collette, B. B. & Luckhurst, B. E. 1999: 316 |
| Schwartz, F. J. 1999: 284 |
| Miller, P. J. 1990: 931 |
| Edwards, A. J. & Glass, C. W. 1987: 659 |
| Robins, C. R. & Ray, G. C. 1986: 246 |
| Lubbock, R. 1980: 296 |
| Randall, J. E. 1968: 248 |
| Bohlke, J. E. 1953: 113 |
| Jordan, D. S. 1904: 541 |