Hyalopale zerofskii, Watson & Tilic & Rouse, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4671.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:99459D5F-3C35-4F7D-9768-D70616676851 |
|
persistent identifier |
https://treatment.plazi.org/id/E131C388-1E2B-420C-BE89-AE479E562247 |
|
taxon LSID |
lsid:zoobank.org:act:E131C388-1E2B-420C-BE89-AE479E562247 |
|
treatment provided by |
Plazi (2019-09-18 09:59:31, last updated 2024-11-27 06:40:39) |
|
scientific name |
Hyalopale zerofskii |
| status |
sp. nov. |
Hyalopale zerofskii View in CoL sp. nov.
urn:lsid:zoobank.org:act:E131C388-1E2B-420C-BE89-AE479E562247
Figs 1F View FIGURE 1 ; 5 View FIGURE 5 A–F, 6A–G
Material examined. Holotype: SIO-BIC A8084, Eastern Pacific Ocean, California, San Clemente Island , 32°54’59.25”N, 118°27’50.67”W, maerl, 10 m., coll. Phil Zerofski, August 2, 2017, 1, 17E, L: 1.7mm, W: 0.9mm, ovigerous female. GoogleMaps
Paratypes: SIO-BIC A8083 same collecting details as holotype; 3, (1, 18E, L: 1.25mm, W: 0.45mm, ovigerous female; 1, 15E, ovigerous female) ; SIO-BIC A8082, Eastern Pacific Ocean, California, San Clemente Island , same locality details as holotype, maerl, coll. Phil Zerofski, July 20, 2015, 1, 17E, L: 1.25mm, W: 0.45mm, ovigerous female ; SIO-BIC A 10228, ovigerous female, slide series
.
Additional material. NTM W. 29628, Eastern Pacific Ocean , Estacahuite Bay, Oaxaca, Mexico, from Padina algae, shallow depth, coll. Christopher Cruz Gomez, 25 Aug., 2007, 1 anterior body fragment, 9NE ; NTM W. 23735, Eastern Pacific , Moorea, French Polynesia, west side of fore-reef, hook inside, Stn. M 531, 1m, coral rubble, November 2010, coll. J. Thomas (Moorea Biocode), 1, 15E, L: 1.1mm, W: 0.6mm, (note: 11 anterior segments used up for DNA which failed, 4 posterior segments registered) .
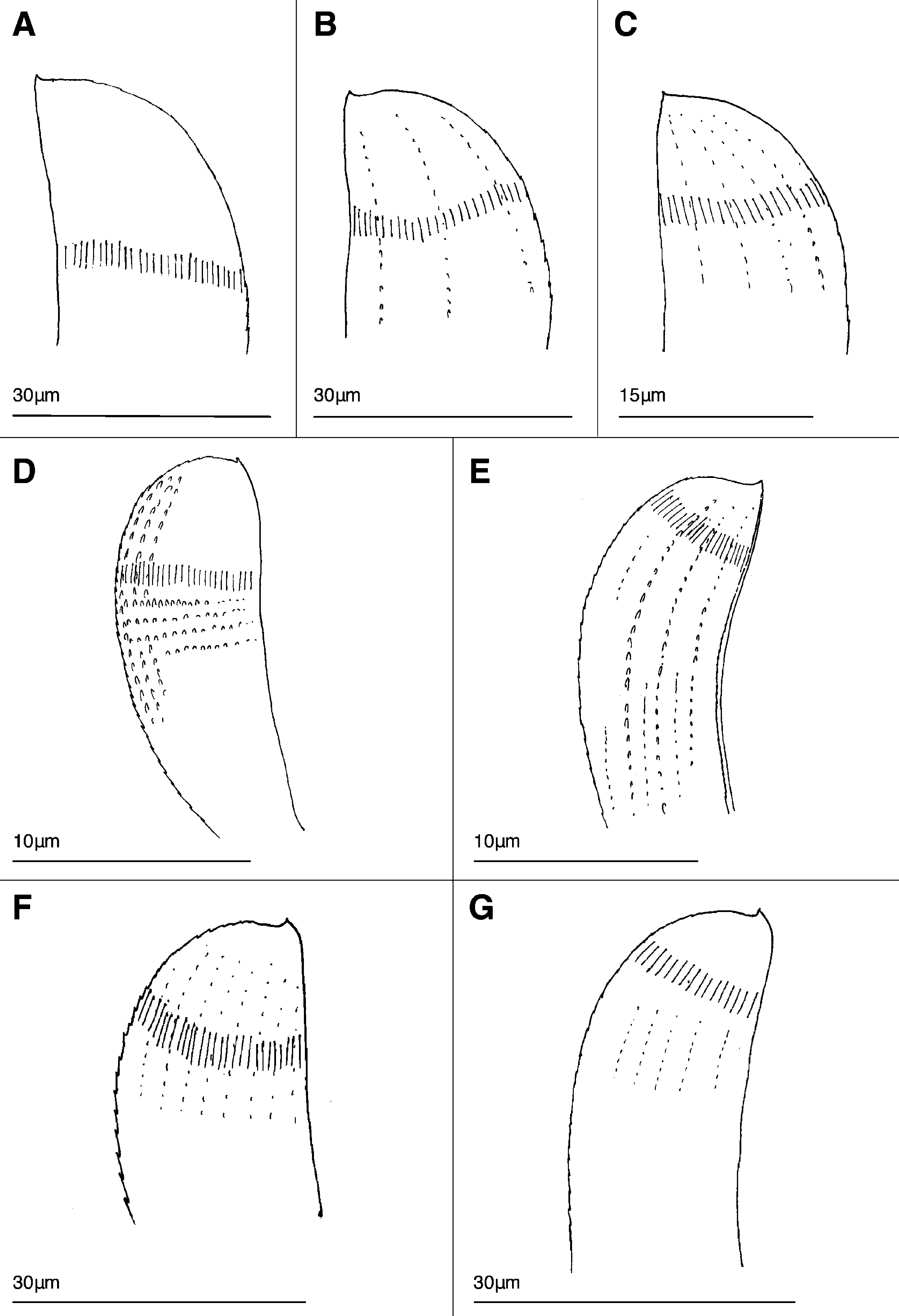
Diagnosis. Hyalopale with mid-body main paleae with rounded brow, 25/26 (27) ribs, multiple shallow raised ribs.
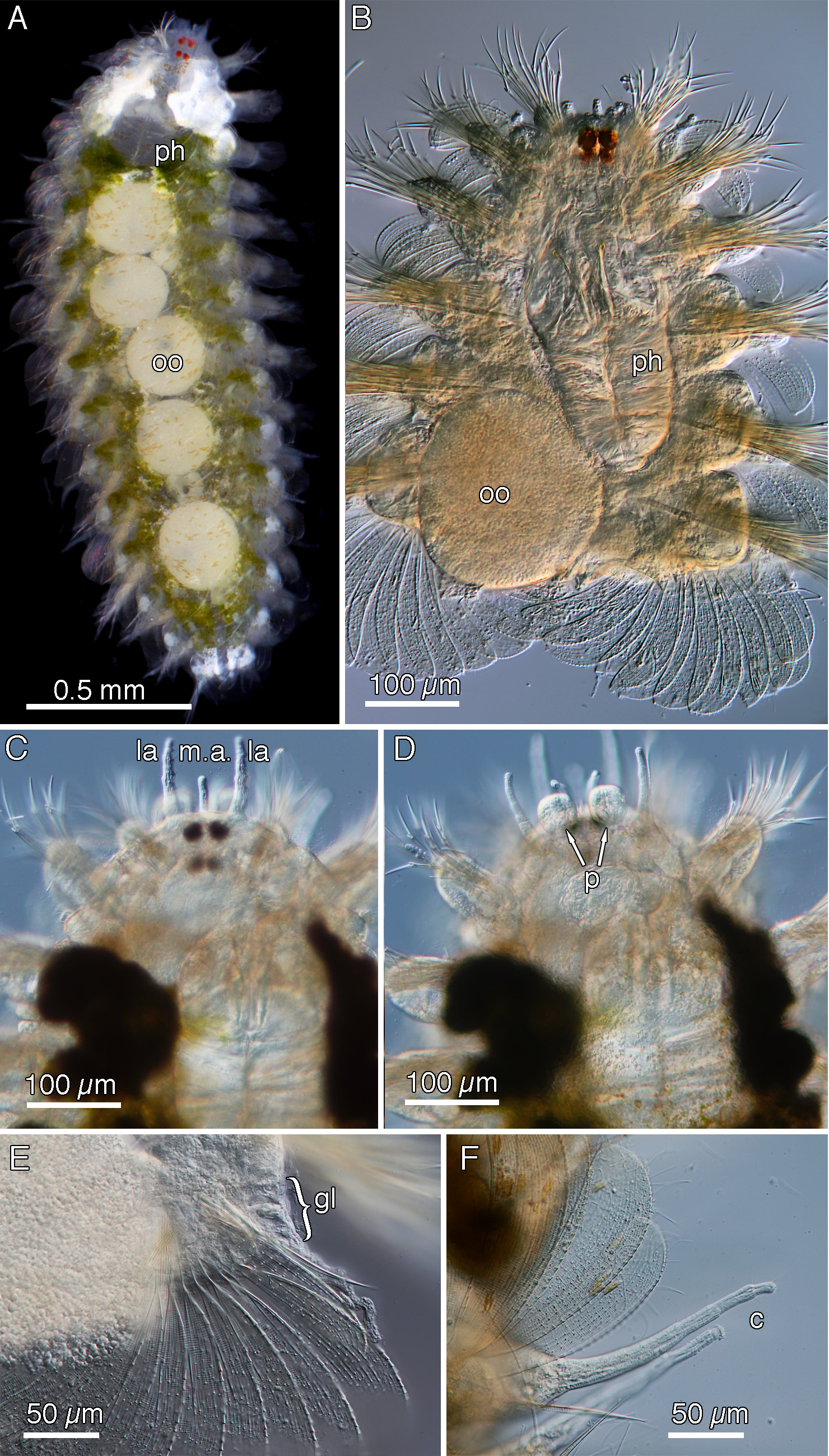
Description (based on holotype, paratypes where noted). Live ovigerous female of 17 segments with two pairs of large cherry-red eyes, white pigmentation present dorsally in five anterior segments, green-brown internal body pigment visible; with five large eggs over segments 7–13, smaller eggs also present ( Fig. 5A View FIGURE 5 ); smallest paratype ovigerous female of 15 segments with three large eggs plus smaller eggs. Gametous paratypes also possess light to dark green internal pigmented cells. Prostomium with slender finger-shaped median and two lateral antennae, two rounded palps ventrally, segment 1 with two pairs of slender tentacular cirri; segment 11 with six notochaetal spines; barrel shaped pharynx with pair pointed stylets situated close together with longitudinal groove facing inwards ( Fig. 5 View FIGURE 5 B–D). Mid-body notopodium with one subacicular curved lateral spine with minute serration in part on both margins; main paleae number 11 with 25–26 (27) and two counts of 28 ribs and 12–14 very finely raised ribs (~8 more obvious, Fig. 1F View FIGURE 1 ); on high magnification horizontal striae are relatively widely spaced. Lateral-most and midline-most main paleae smaller with 21–23 ribs; segment 17 has two midline-most smaller main paleae, slightly symmetrical in shape, 16–18 ribs ( Fig. 6E View FIGURE 6 ). Midline spines absent. Main paleae with slightly rounded to sloping brow, tiny upright apex, inner margin with minute serrations, convex margin with visible serration becoming minutely serrated on brow. Notopodia with slender dorsal cirrus, style extending at least half to 2/3 length of paleal fan, cilia tufts laterally and distally on dorsal cirri; rounded swollen glands visible posterior to dorsal cirrophore ( Fig. 5E, F View FIGURE 5 ). Mid-body neuropodia with 4 (5) superior long, very slender spinigerous articles; four mid- superior very slender falcigers; mid-group number 14–18 comprise upper slender falcigers and lower slightly broader falcigers with visible blade serration; inferior group with 4–6 slender shorter blades. Ventral cirri length as long as distance to neuropodial tip.
Remarks. Morphology. Paleal rib counts down the body of the holotype include: anterior chaetigers, segment III, has 18 ribs, segment V with 24 ribs, mid-body chaetigers has 25, 27 (28) ribs and posterior chaetigers, segment 15 has 24–26 ribs and segment 17 possesses 2–3 midline-most smaller main with 16–20 ribs. The possible Hyalopale zerofskii sp. nov. Mexican individual from Estacahuite Bay, Oaxaca, is a smaller specimen compared to the southern Californian type material and has no sign of gametes. The original specimen was 13E; the anterior body of the examined specimen has 9 segments (L: 0.8mm, W: 0.5mm); it has a broad tapered pharynx and posterior caeca, a mouth fold with large mouth papillae and the rounded tips of the two stylets visible. Larger and slightly smaller main paleae including the smaller midline-most main, exhibit a similar shape and range of internal and raised ribs that agrees with that observed with the type material of H. zerofskii sp. nov.
The possible Hyalopale zerofskii sp. nov. specimen from Moorea ( French Polynesia) possesses a mid-body notopodium comprising: lateral spine with minute serration in part on both margins; main paleae with tiny upright apices, relatively widely spaced horizontal striae, 21–26 internal ribs (lateral-most main paleae with 21 ribs), 5–6 shallow raised ribs; midline spines absent. Posterior-most notopodium, segment 15, with three main paleae: two with 18 ribs and midline-most main palea, almost symmetrical, with 15 ribs. Although H. zerofskii sp. nov. from Moorea has main paleae with apices more ‘swept-up’ compared to the more ‘snub’ apices of main paleae in the southern California and Mexican specimens, it exhibits similarity of main paleae shape and number of ribs and is therefore identified within a Hyalopale zerofskii ‘ species complex’.
Hyalopale zerofskii sp. nov. lacks midline spines and has a paleal shape and degree of raised ribs quite different to that of the Western Atlantic H. bispinosa s.s (cf Fig. 1F View FIGURE 1 with 1A, B). Morphological and molecular results indicate that H. zerofskii sp. nov. from the eastern Pacific is distinct from the Caribbean Sea species and is the sister group (though poorly supported) to H. sapphiriglancyorum sp. nov. from the western Pacific (cf Fig. 1F & G View FIGURE 1 ; Fig. 13 View FIGURE 13 ).
Pigmentation & epibionts. Hyalopale zerofskii sp. nov. has dense white pigmented cells of the dorsal anteriormost segments in two lateral ‘shield’ shaped areas and internal light to dark green/brownish pigmented body cells in all live material ( Fig. 5A View FIGURE 5 ). Both forms of pigmentation are seen in the majority of other Hyalopale species e.g. the Caribbean H. bispinosa s.s. ( Fig. 2 View FIGURE 2 ) and the West Pacific species H. sapphiriglancyorum sp. nov. ( Fig. 7A View FIGURE 7 ).
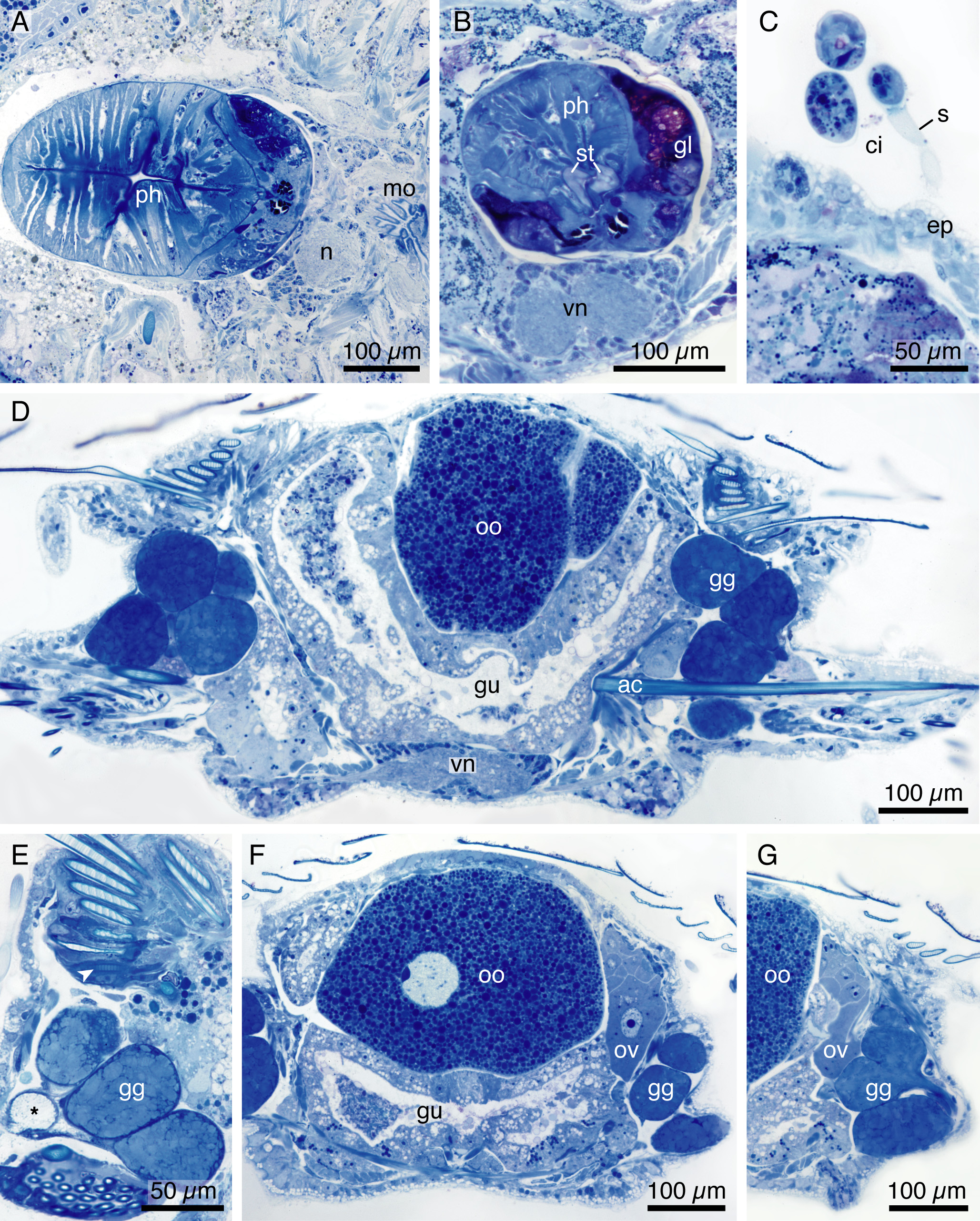
All four of the sectioned specimens were ovigerous females. Multiple large vitellogenic oocytes (± 300–400µm in diameter) were observed dorsally in the coelomic cavity ( Fig. 6D, F View FIGURE 6 ). Oogenesis occurs in laterally positioned ovaries, where multiple non-vitellogenic oocytes could be seen ( Fig. 6F, G View FIGURE 6 ). The green/brown globular material visible through the live animal’s body wall is likely associated with the reproductive system, due to its close proximity to oocytes ( Fig. 6 View FIGURE 6 D–G). Though the function still remains unknown and needs further investigation, we hypothesize that these are glandular secretions. This is corroborated by their vesicular appearance, and the lack of identifiable nuclei and organelles. In certain sections, it was observed that the dark homogenous contents of these structures were emptied near the parapodia, leaving behind an empty vesicle ( Fig. 6E View FIGURE 6 ). Developing paleae are located in the ventral rim of the paleal fan ( Fig. 6E View FIGURE 6 ). The muscularized pharynx is equipped with large pharyngeal glands anteroventrally ( Fig. 6A, B View FIGURE 6 ).
Epibiont ciliates identified as loricate peritrichs are attached to the epidermis aborally by a stalk, which are then covered by the paleae ( Fig. 6C View FIGURE 6 ). Epibiont ciliates have been recorded in a number of different taxa of the Syllidae attached to intersegmental furrows, dorsal and ventral surfaces, nuchal organs, mouth opening and anterior cirri and are known to thrive in oxygen poor waters (Campos et al. 2014).
Etymology. This species is named for Phil Zerofski, Experimental Aquarium Manager, Marine Technician/Collector at Scripps Oceanography. He has been a great friend of the Rouse lab and brought us many wonderful specimens over the years and we honor him with this new species.
FIGURE 1. Composite plate comparing notochaetal main paleae in Hyalopale species. A. Hyalopale bispinosa s.s. Florida, W. Atlantic; B. H. cf. bispinosa Crete, Mediterranean Sea; C. H. leslieae sp. nov. Florida Keys, W. Atlantic; D. H. furfuricula sp. nov. Mozambique, W. Indian Ocean; E. H. angeliensis sp. nov. Western Australia, E. Indian Ocean; F. H. zerofskii sp. nov. California, E. Pacific; G. H. sapphiriglancyorum sp. nov. E. Indonesia, W. Pacific. Note: correct number of raised and internal ribs figured.
FIGURE 2. Hyalopale bispinosa s.s. Bahama Islands, LACM–AHF 2879, live, colour photo, by Leslie Harris.
FIGURE 5. H. zerofskii sp. nov., A–F, San Clemente Is. California, Paratypes, SIO-BIC A8083. A. live, colour stereo micrograph, ovigerous female; B–F. micrographs. B. anterior end, dorsal; C. anterior end, dorsal; D. anterior end, ventral; E. midbody notopodium in part with glands; F. detail of dorsal cirri with cilia.
FIGURE 6. H. zerofskii sp. nov., San Clemente Is. California, SIO-BIC A10228, A–G. Histology: A. horizontal section, B–G. cross sections. A. pharynx (ph) with circumoesophageal ganglion (n) and mouth (mo); B. pharynx (ph) with stylets (st) and prominent pharyngeal glands (gl); C. epibiont ciliates (ci) with stalk (s) attached to epidermis (ep); D. parapodium with ventral nerve (vn), gut (gu), oocyte (oo), green globules (gg), ventral acicula (ac); E–G. elaboration of D, including developing palea in chaetal follicle (arrow), ovary (ov), *empty vesicle.
FIGURE 7. H. sapphiriglancyorum sp. nov. Raja Ampat, Indonesia, Paratypes, MZB Poly. 00410, live, colour stereo micrograph, gametous individuals.
FIGURE 13. Maximum likelihood tree obtained with the molecular combined data (COI, 16S, 18S and H3) and with the hesionid Nereimyra punctata as outgroup. Values at nodes are shown as ML bootstrap then MP jackknife values. * =>90% values for ML bootstrap and MP jackknife values. The single MP tree was identical except that it recovered Paleanotus as the sistergroup to the remaining Chrysopetalinae, rather than to Hyalopale, though with only 53% jackknife support.
| NTM |
Northern Territory Museum of Arts and Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Chrysopetalinae |
|
Genus |
1 (by plazi, 2019-09-18 09:59:31)
2 (by ExternalLinkService, 2019-09-18 10:09:49)
3 (by ExternalLinkService, 2019-09-18 10:33:23)
4 (by plazi, 2019-09-19 14:19:45)
5 (by angel, 2019-09-27 13:19:38)
6 (by veselin, 2019-10-02 12:42:37)
7 (by ExternalLinkService, 2021-10-29 02:07:07)
8 (by ExternalLinkService, 2021-10-29 05:09:53)
9 (by plazi, 2023-10-30 19:06:09)