Rhopalurus crassicauda Caporiacco, 1947
|
publication ID |
https://doi.org/ 10.18590/euscorpius.2008.vol2008.iss70.1 |
|
publication LSID |
lsid:zoobank.org:pub:2DF716C7-F789-41EA-9050-8E5C11D4A47C |
|
DOI |
https://doi.org/10.5281/zenodo.4673074 |
|
persistent identifier |
https://treatment.plazi.org/id/6F3187EF-FFED-FFC3-FCCF-9F2CFB42F8A4 |
|
treatment provided by |
Carolina |
|
scientific name |
Rhopalurus crassicauda Caporiacco, 1947 |
| status |
|
Rhopalurus crassicauda Caporiacco, 1947
Figures 5–9 View Figure 5 View Figure 6 View Figure 7 View Figure 8 View Figure 9
Rhopalurus crassicauda Caporiacco, 1947: 20 ; Caporiacco, 1948: 609–610, figs. 1–3; Lourenço, 2002: 36, 98–100, 110–111, figs. 214–224; Teruel, 2006: 51–52.
Rhopalurus laticauda pintoi: Lourenço, 1982: 107–108 , 115, 117–119, 121, 134–138; figs. 39–46, 78, table I (misidentification); Fet & Lowe, 2000: 220–221 (misidentification).
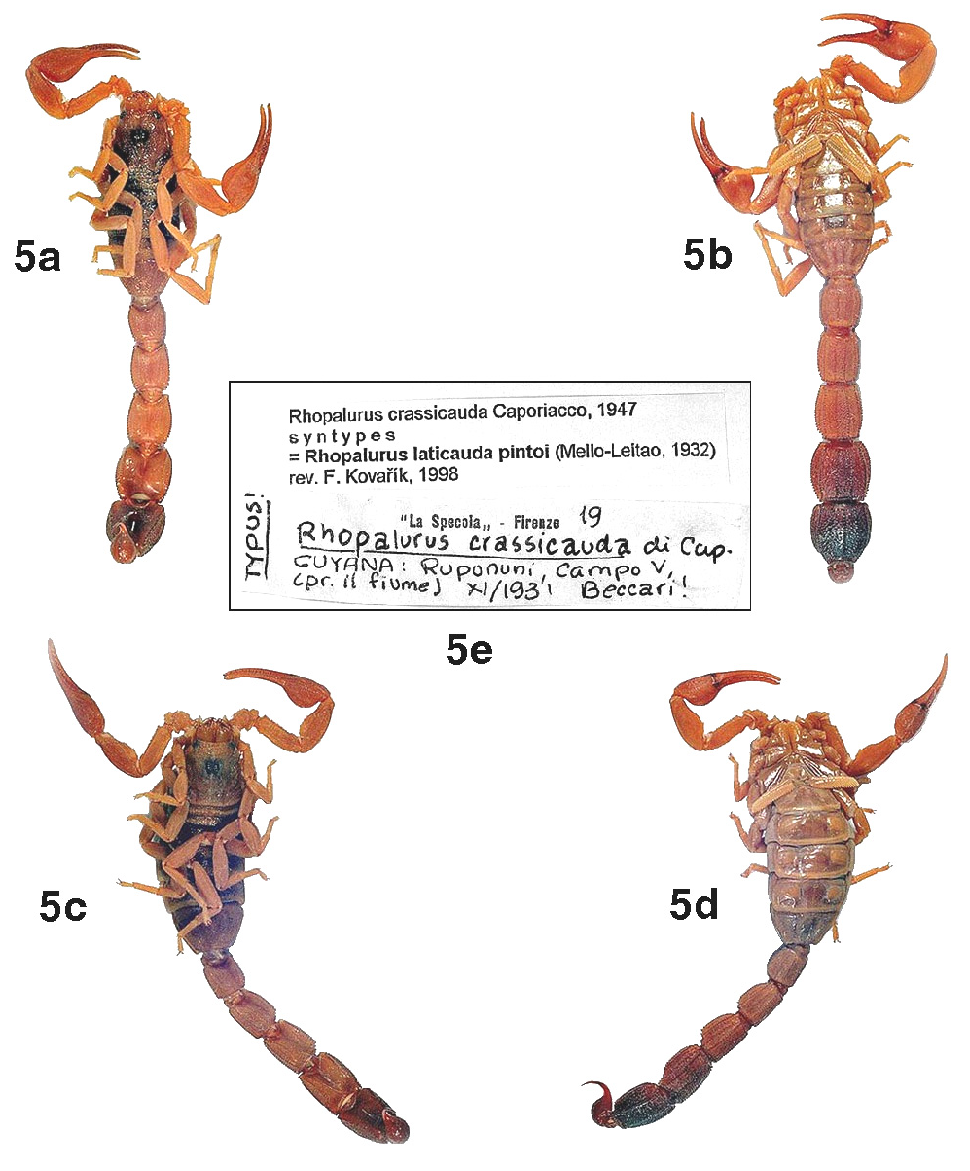
Type data: 2 adult ♂♂ and 1 adult ♀ syntypes ( MZUF, indirectly examined; see Remarks and Fig. 4 View Figure 4 ): Guyana, Rupununi , Campo V (near the river), November 1931, Beccari .
Diagnosis: species of moderately small size (males 40–45 mm, females 45–50 mm) for the genus. Body light brown, densely infuscate on carapace, tergites I–VI, pedipalp fingers, metasomal segment V and telson; metasoma ventrally with a wide and solid blackish stripe. Pedipalp chelae robust in both sexes, more conspicuously in males; fingers without basal lobe/notch combination, but with moderate scallop in adult males; fingers with eight principal rows of granules, flanked by a few supernumerary granules. Sternite III and pectines with stridulatory apparatus slightly reduced; sternite V with a vestigial smooth patch in males. Metasoma distally incrassate on both sexes, much more conspicuously in males; telson vesicle small, subaculear tubercle moderate, spinoid and far removed from the base of aculeus. Pectines with 24–25 teeth in males, and 20–22 in females.
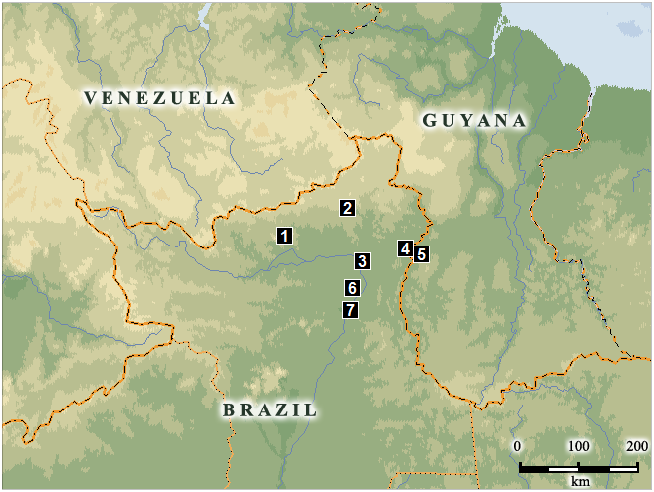
Distribution ( Fig. 7 View Figure 7 ): this species appears to be endemic the Rupununi region comprising the border region of Brazil, Guyana, and Venezuela. Specimens have been collected only from two localities in Brazil and one in Guyana, but it is probably present also in neighboring Venezuela (southern Bolivar State), where there is similar potentially suitable habitat.
Ecological notes: this species lives together with R. pintoi , but according to the personal notes of the collector at least at Rupununi both species appear to avoid interspecific competition by habitat segregation: R. crassicauda is very common in the open grasslands where it mostly hides inside soil crevices, but only one specimen was found in the relict forested patches to which R. pintoi is restricted.
MATERIAL EXAMINED: Guyana, Southwestern , Rupununi, March 2008, H.-W. Auer, 1 adult ♀ topotype ( RTO: Sco.0384), 2 adult ♀♀ topotypes ( AKT) .
Remarks: The Brazilian female specimen illustrated as an adult twice by Lourenço (1982: figs. 27–28; 2000: fig. 215) is in fact a juvenile, which is evident from its slender pedipalp chelae (i.e., compare these pictures to Figures 5c–d View Figure 5 of the present paper). We could not obtain the types of R. crassicauda for the present study, but we were fortunate to get three adult topotypes and also to receive a great help from František Kovařík, who personally examined two syntypes in 1998 and kindly provided us with high–resolution color photos of both specimens and its original label.
The identity of R. crassicauda cannot be considered as fully clarified yet. It was regarded as a junior synonym of R. laticauda by Lourenço (1982), still retaining its validity at subspecific rank under the incorrectly applied name R. laticauda pintoi (see above). Here we have demonstrated that Lourenço's previous interpretation of R. pintoi was erroneous, and that it is a valid species totally different from R. crassicauda (see above), but in turn, R. crassicauda is virtually indistinguishable from R. laticauda (see its updated diagnosis in Teruel & Roncallo, 2008). This strongly suggests that both taxa may indeed be conspecific, but we refrain from proposing the formal synonymy here because adequate samples of R. laticauda are still unavailable to us. In addition, the known distribution of both taxa still appears allopatric, with R. laticauda in the Orinoquian Llanos and R. crassicauda in the northern reaches of the Amazon basin. Nevertheless, this apparent allopatry requires rigorous confirmation because it may only reflect poor and/or inadequate sampling in the intervening lowland areas, which otherwise seem to lack any real geographic barrier for scorpion dispersal. These areas are covered mainly by seasonally inundated forests, but other species of Rhopalurus are known to be well adapted to similar habitats and escape from flooding by readily climbing onto the vegetation, as does the Cuban endemic R. junceus in the extensive swamps of Zapata, Cauto and north–central Camagüey (R. Teruel, unpublished data); even this same strategy has also been described for other terrestrial arachnids of the Amazon basin such as schizomids (Cokendolpher & Reddell, 2000). For the moment, we retain R. crassicauda as valid but its true identity warrants further studies.
| MZUF |
Museo Zoologico La Specola, Universita di Firenze |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhopalurus crassicauda Caporiacco, 1947
| Teruel, Rolando & Tietz, Alexander K. 2008 |
Rhopalurus laticauda pintoi: Lourenço, 1982: 107–108
| LOURENCO 1982: 108 |
Rhopalurus crassicauda
| TERUEL 2006: 51 |
| LOURENCO 2002: 36 |
| CAPORIACCO 1948: 609 |
| CAPORIACCO 1947: 20 |