Leucetta floridana, (HAECKEL, 1872)
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2009.00522.x |
|
DOI |
https://doi.org/10.5281/zenodo.5748135 |
|
persistent identifier |
https://treatment.plazi.org/id/6C011708-2A29-D704-FCEB-F9F36F3B0193 |
|
treatment provided by |
Carolina |
|
scientific name |
Leucetta floridana |
| status |
|
LEUCETTA FLORIDANA ( HAECKEL, 1872) View in CoL ( FIG. 5 View Figure 5 )
Synonymies: Leucaltis floridana Haeckel, 1872: 144 , pl. xxvi, figures 1–17, pl. xxvii, figure 1 (original description).
Leucetta floridana View in CoL ; Dendy & Row, 1913: 734 (generic reallocation); Burton, 1963: 46, 252–253, figure 118 (proposed as junior synonym of L. microraphis Haeckel, 1872 View in CoL .
Leucetta microraphis View in CoL ; Borojevic & Peixinho, 1976: 1003–1005, figure 9 ( L. floridana View in CoL after Borojevic & Klautau, 2000).
Leucetta aff. floridana View in CoL ; Lehnert & van Soest, 1998: 99, figure 24.
Leucilla floridana ; Jenkin, 1908: 453 (to be verified sensu Borojevic & Klautau, 2000; the description of that material does not allow its identification).
Leucetta floridana View in CoL ; de Laubenfels, 1950: 146, figure 64, pl. II (fig. 8) ( L. microraphis View in CoL after Borojevic, 1967).
Type material: Haeckel’s specimens are lost fide Burton (1963).
Type locality: Coast of Florida. Collector A. Agassiz.
Reported distribution: Florida ( Haeckel, 1872), Bermuda ( de Laubenfels, 1950), Jamaica (Lehner & van Soest, 1998), Brazil: Ceará, Rio Grande do Norte, Rocas Atoll ( Borojevic & Peixinho, 1976), Wasin (eastern Africa; Jenkin, 1908, to be confirmed).
Analysed material: – Bocas del Toro ( Panama), PC BT 12, 22, 23 – San Andrés Island ( Colombia), UFRJPOR 5363: Leeward-reef, ‘ West View’, fossil wave-cut notch, 5 m of depth, coll. D. Valderrama, xi.2000 ; UFRJPOR 5364, 5365, 5366, 5367: ‘ La Piscinita’ , fossil wave-cut notch, 2–5 m of depth, coll. D. Valderrama, xi.2000 . – Urabá ( Colombia), UFRJPOR 5356: Sapzurro, ‘ Bajo El Palmar’, inclined reef slope, 15 m of depth, coll. D. Valderrama, ii.2004 ; UFRJPOR 5357, 5358, 5359, 5360: ‘ Bajo Agua Viva’ , reef terrace, 15 m of depth, coll. D. Valderrama, ii.2004 ; INV-POR 583 (a fragment also in UFRJPOR 5362): reef base, 16 m of depth, coll. S. Zea, ix.1995; INV-POR 542 (a fragment also in UFRJPOR 5361): Cabo Tiburón , reef terrace, 9 m of depth, coll. S. Zea, ix.1995 . – Ceará ( Brazil), MNRJ 8440 View Materials , 8445 View Materials , 8465 View Materials , 8481 View Materials : Trawling, Station 30 . – Rio Grande do Norte ( Brazil), BPOTPOR 201, 202: Trawling 4, Station 4, xi.2003 ; BPOTPOR 540, 610: Risca das Bicudas, 10 m of depth, coll. F. Moraes and G. Muricy, iii.2007 ; BPOTPOR 634: Urca do Tubarão , 8 m of depth, coll. G. Muricy, iii.2007 . – Rocas Atoll ( Brazil), MNRJ 7630 View Materials , 7648 View Materials , 7725 View Materials : Barretinha, 12 m of depth, coll. E. Hajdu, F. Moraes and M. Oliveira, xi.2003 . – Fernando de Noronha Archipelago ( Brazil), MNRJ 8602 View Materials : Ressurreta, 4 m of depth, coll. F. Moraes, viii.2004 ; MNRJ 8609 View Materials : Ilha Sela Gineta , 7 m of depth, coll. F. Moraes, viii.2004 . – Abrolhos ( Brazil), UFRJPOR 4703: Parcel das Paredes, 8 m of depth, coll. G. Muricy, x.1997 .
Suggested distribution: Florida ( Haeckel, 1872), Jamaica ( Lehnert & van Soest 1998), Brazil: Pará, Ceará, Rio Grande do Norte, Rocas Atoll, Paraíba, Pernambuco, Alagoas, Sergipe, Bahia, Espírito Santo ( Borojevic & Peixinho, 1976). In addition: Colombia (Urabá and San Andrés Island), Brazil (Fernando de Noronha Archipelago).
Description
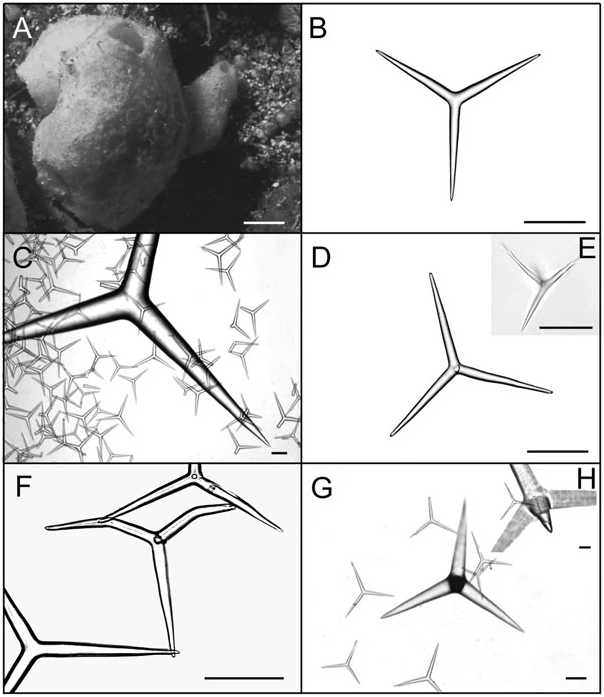
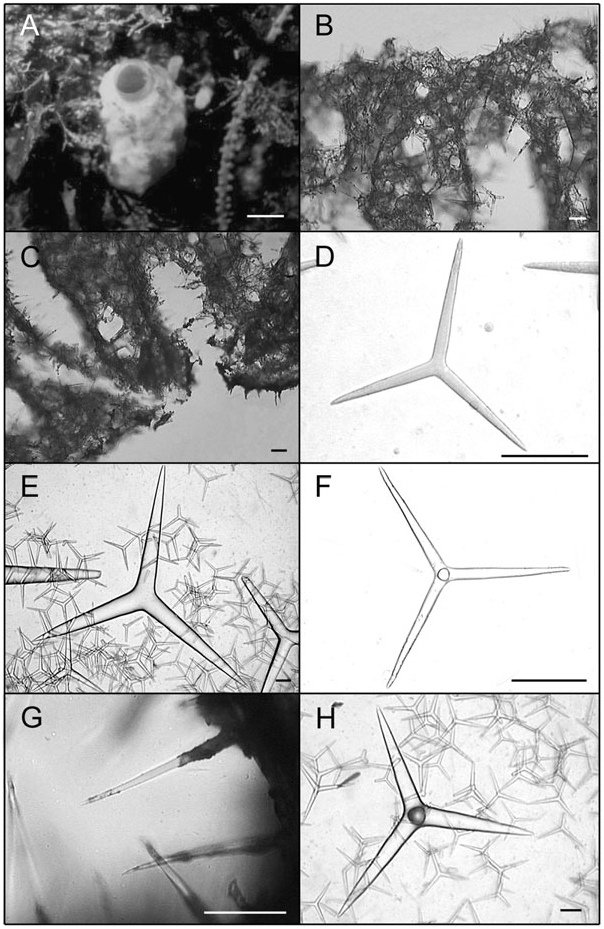
Solitary or grouped globular to pyriform tubes ( Fig. 4A View Figure 4 , 5A View Figure 5 ). Individuals may be highly deformed when encrusting small crevices. In photophylous environments, its colour is light blue. After preservation in ethanol colour becomes beige to dark brown. Surface is rough and, in high wave energy environments, tends to be hispid because of the high spicule content. Consistence is always firm, varying from friable to hard. The osculum is localized at the top of the body. In deformed individuals, one to several oscula are localized at the top of apical projections. Below each osculum there is a wide atrial cavity, always hispid because of the apical actine of tetractines I. Numerous exhalant canals are dispersed in the atrium. In individuals with two or more oscula, wide canals may interconnect different atrial cavities. The aquiferous system is leuconoid and the skeleton is disorganized, as typical of the genus. The cortex and the atrial wall are thin, whereas the choanosome is thick. Triactines II and tetractines II are concentrated in the cortex and lie tangentially to the surface, with the apical actine of tetractines penetrating the choanosome. Those spicules give a smooth appearance to the sponge. Subcortical holes may be present in abundance, and inhalant and exhalant canals are always profuse. Triactines I and tetractines I form the walls of subcortical holes and choanosomal canals, being tangentially aligned and densely packed around them. The apical actine of such tetractines conspicuously protrudes into exhalant canals. Triactines I also form an irregular meshwork along the entire body wall. The atrial wall is formed by triactines I and tetractines I tangentially aligned and densely packed around the atrium, projecting their apical actines into the atrial cavity and giving it a hispid appearance ( Fig. 5B, C View Figure 5 ).
Spicules: Triactines I. These spicules are the most abundant. They are similar in shape to triactines II, although sagittal spicules may also be found. They are abundant in the choanosome, but sagittal spicules are mainly found tangentially aligned and densely packed around subcortical holes, choanosomal canals and the atrium [105.6–143.3 (±28.7) - 217.8/9.9–17.1 (±4.9) - 33.0 Mm] (N = 30) ( Figs. 4B View Figure 4 , 5D View Figure 5 , 7A, B View Figure 7 ).
Triactines II. They are regular, equiradiate, and equiangular. Actines are conical, with slightly sharp tips. Most lay tangentially to the surface and their size is very variable. Few can be found scattered in the choanosome, laying perpendicular to the atrium [257.4–696.2 (±279.7) - 1181.5/33.0–102.1 (±46.2) - 194.6 Mm] (N = 30) ( Figs. 4C View Figure 4 , 5E View Figure 5 , 7C, D View Figure 7 ).
Tetractines I. The basal system of these spicules is similar to that of triactines I. Apical actines are conical and smooth, with slightly sharp tips. They are straight or often undulated, with a single bend near the tip. Most tetractines I are tangentially aligned and densely packed around subcortical holes, choanosomal canals and the atrium. Nevertheless, apical actines only protrude conspicuously into exhalant canals and into the atrium. Very rarely, they are scattered in the choanosome, always in proximity to the canals, laying perpendicularly to the atrium. Sagittal tetractines may also be found [105.6–137.4 (±24.1) - 224.4/9.9–15.4 (±3.6) - 26.4 Mm] (N = 30) ( Figs. 4D–F View Figure 4 , 5F, G View Figure 5 , 7E, F View Figure 7 ).
Tetractines II. The basal system of these spicules and their distribution are similar to those of triactines II. These spicules can be abundant, rare or even be absent. Apical actines are conical, straight and smooth, and penetrate the choanosome [278.0–665.5 (±301.0) - 1042.5/48.7–102.5 (±51.3) - 180.7 Mm] (N = 8) ( Figs 4G, H View Figure 4 , 5H View Figure 5 , 7G, H View Figure 7 ).
Ecology and biogeography: Leucetta floridana can be found in semishadowed environments in reef terraces and slopes, where it can be encrusting in small crevices or erect under overhangs and on vertical slopes. This species seems to have a patchy distribution within a reef and is in general rare. It does not show any sign of predation or fouling. Nonetheless, if hard substrate is limited, it enters into direct contact with other organisms (i.e. corals and other sponges), when it shows external morphological alterations but no sign of tissue injury.
The presence of L. floridana in the Caribbean and Brazil provides new support for the existence of a sole zoogeographical province in the western tropical Atlantic. The Amazon River outflow penetrates 500 km offshore and 30 m deep ( Rocha, 2003). Such discharge of freshwater could represent a significant barrier to gene flow between Caribbean and Brazilian populations. However, as observed for some other marine species ( Lazoski et al., 2001; Rocha, 2003; Wörheide et al., 2005), L. floridana seems capable of maintaining gene flow between the two areas. There are no studies on the reproduction of Leucetta species, consequently we do not know if larvae of this genus have a long duration or not. Studies on reproduction of calcareous sponges that measured time to larval settlement showed that it takes from a few hours to a maximum of three days ( Minchin, 1896; Amano & Hori, 2001; Leys & Eerkes-Medrano, 2005). Hence, it is unlikely that larvae of L. floridana can cross the Amazon barrier between the Caribbean and Brazil. It seems more likely that the species has a continuous distribution including populations below the Amazon plume, in the sponge corridor found by Collette & Rützler (1977). To be sure about this, studies on the reproduction of L. floridana and collections under the Amazon plume should be conducted.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Leucetta floridana
| Valderrama, Diego, Rossi, André Linhares, Solé-Cava, Antonio Mateo, Rapp, Hans Tore & Klautau, Michelle 2009 |
Leucetta aff. floridana
| Lehnert H & van Soest RWM 1998: 99 |
Leucetta microraphis
| Borojevic R & Peixinho S 1976: 1003 |
Leucetta floridana
| de Laubenfels MW 1950: 146 |
Leucetta floridana
| Burton M 1963: 46 |
| Dendy A & Row RWH 1913: 734 |
Leucilla floridana
| Jenkin CF 1908: 453 |