Citrilolepis citrili, Dimitrova & Georgiev & Mariaux & Vasileva, 2019
|
publication ID |
https://doi.org/ 10.1007/s11230-019-09846-y |
|
DOI |
https://doi.org/10.5281/zenodo.5925176 |
|
persistent identifier |
https://treatment.plazi.org/id/654187B3-087D-FF8D-FF8A-FF053D20489B |
|
treatment provided by |
Plazi |
|
scientific name |
Citrilolepis citrili |
| status |
n. g., n. sp. |
Citrilolepis citrili n. g., n. sp.
Type-host: Crithagra citrinelloides ( Passeriformes : Fringillidae ), African citril.
Type-locality: Wondo Genet (7.08°N, 38.63°E), Ethiopia.
Type-material: Three fragmented specimens (coll. 22.xi.2012): 3 anterior fragments consisting of scolex and strobila ending with premature proglottides, and c. 30 fragments of strobila at various stages of maturation (10 slides). The material consisted of highly fragmented specimens and the designation of a complete representative individual as a holotype was not applicable. Syntypes: MHNG-PLAT- 121396 (7 slides), one scolex in Berlese’s medium and fragments of strobila, stained and mounted in Canada balsam; IBER-BAS-C0159.1.4–1.5 (2 slides), one immature specimen and fragments of strobila, stained wholemounts; hologenophore (syntype): MHNG-PLAT- 121395, stained whole-mount.
Site in host: Small intestine.
Prevalence and intensity: In 1 out of 3 birds studied; 3 individuals.
Representative DNA sequence: cox 1 mtDNA, one sequence (GenBank: MK463853 View Materials ).
Etymology: The species name is derived from the common name of the type-host species.
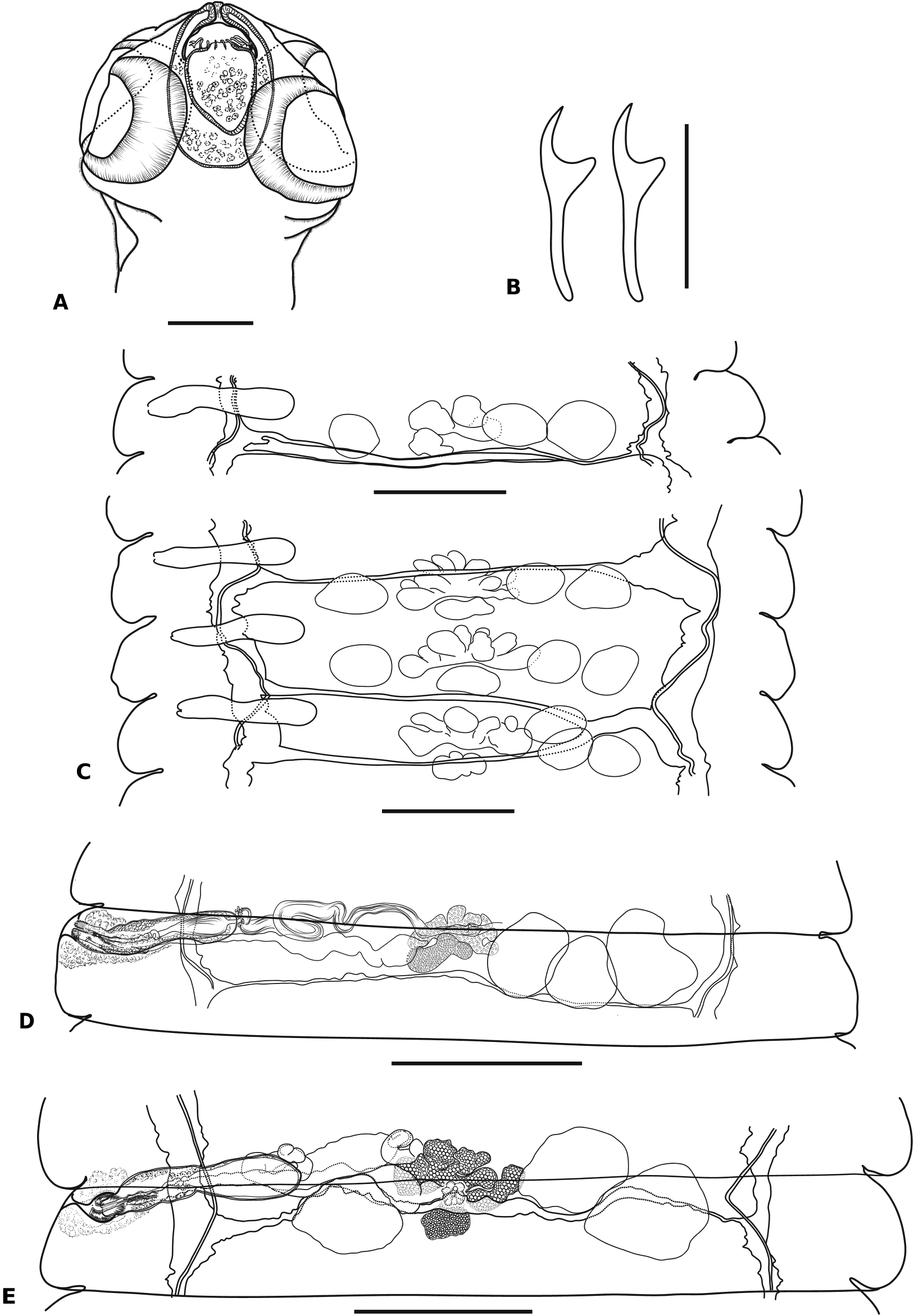
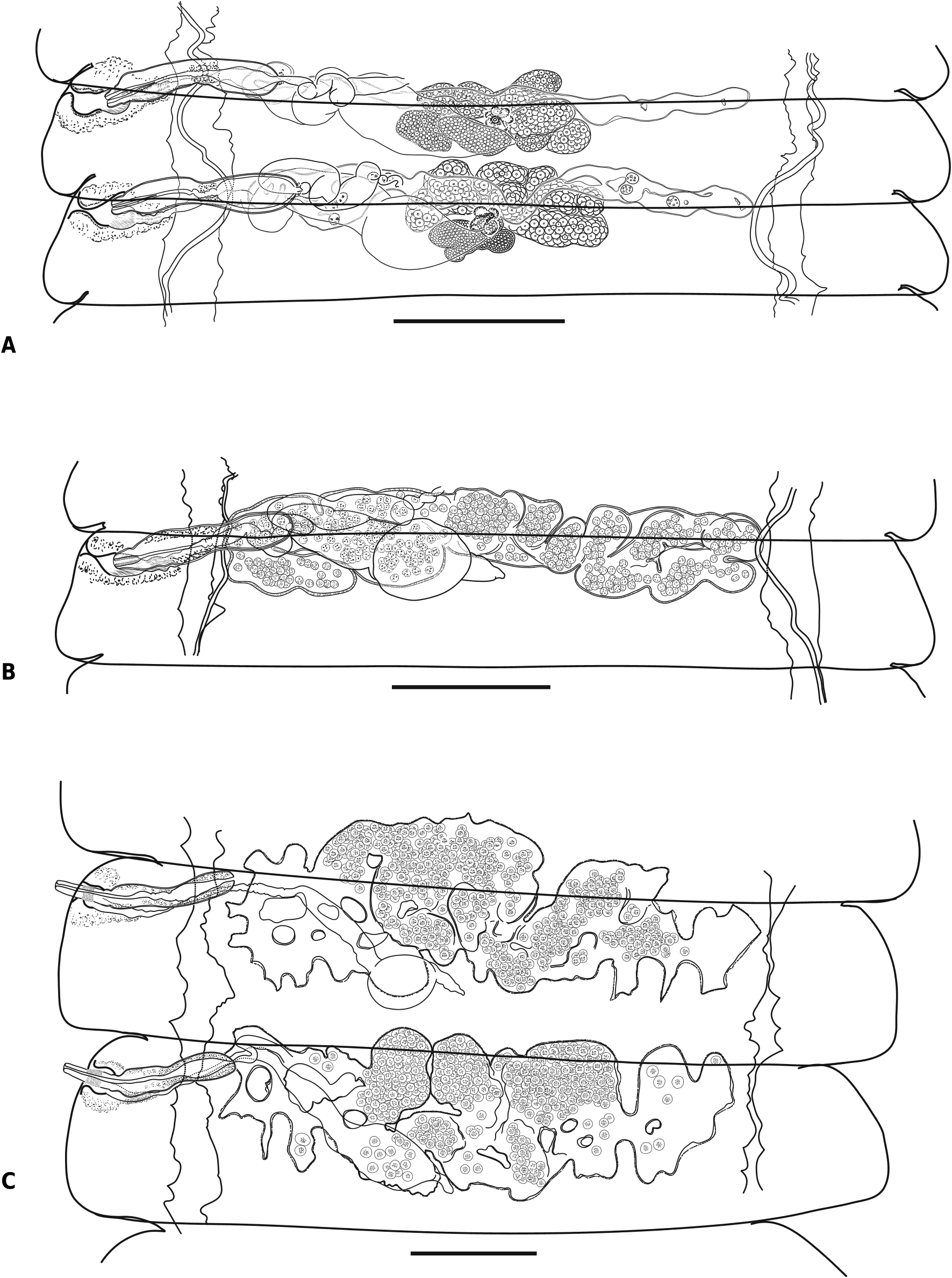
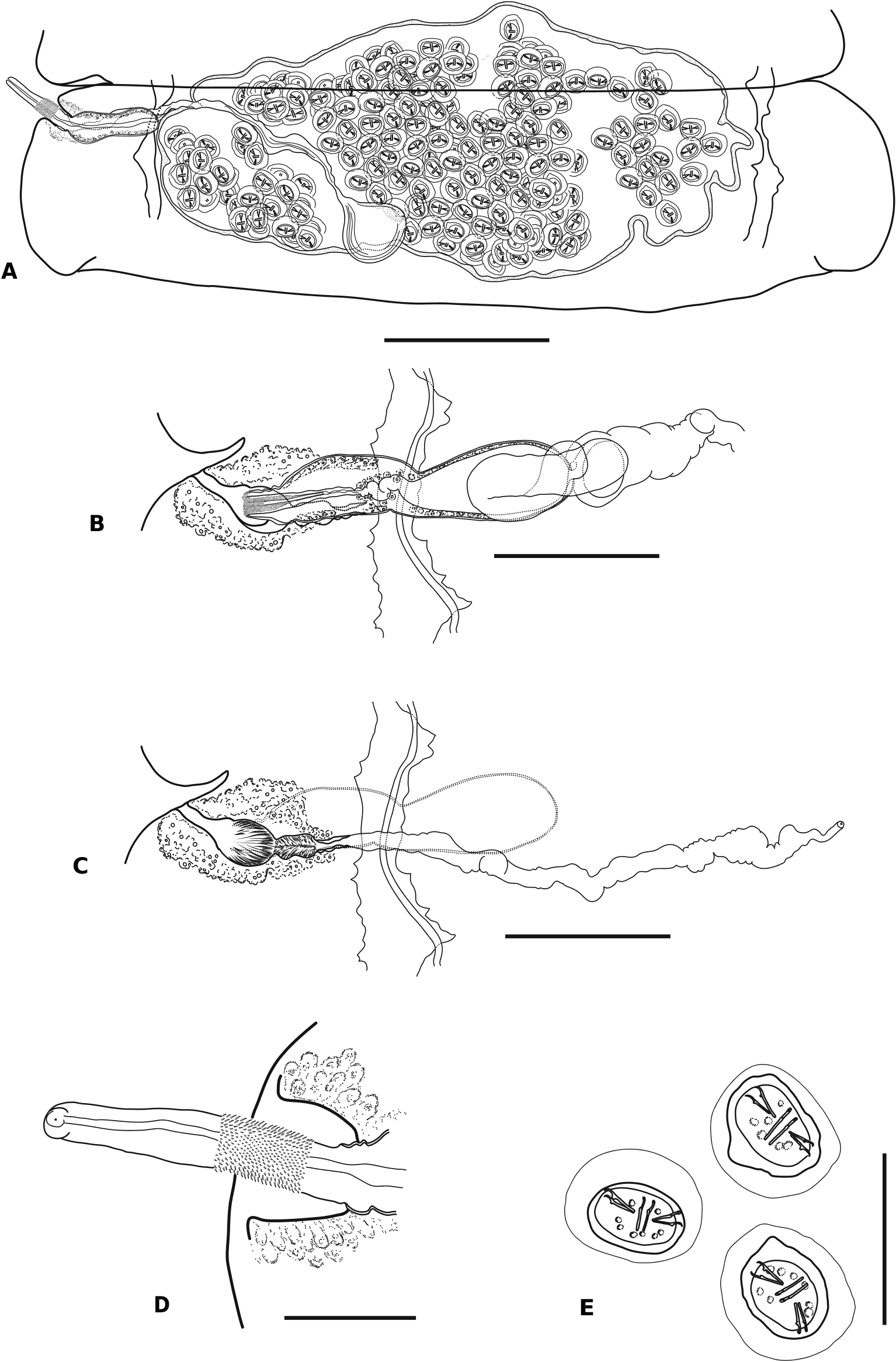
Description ( Figs. 3–6 View Fig View Fig View Fig View Fig )
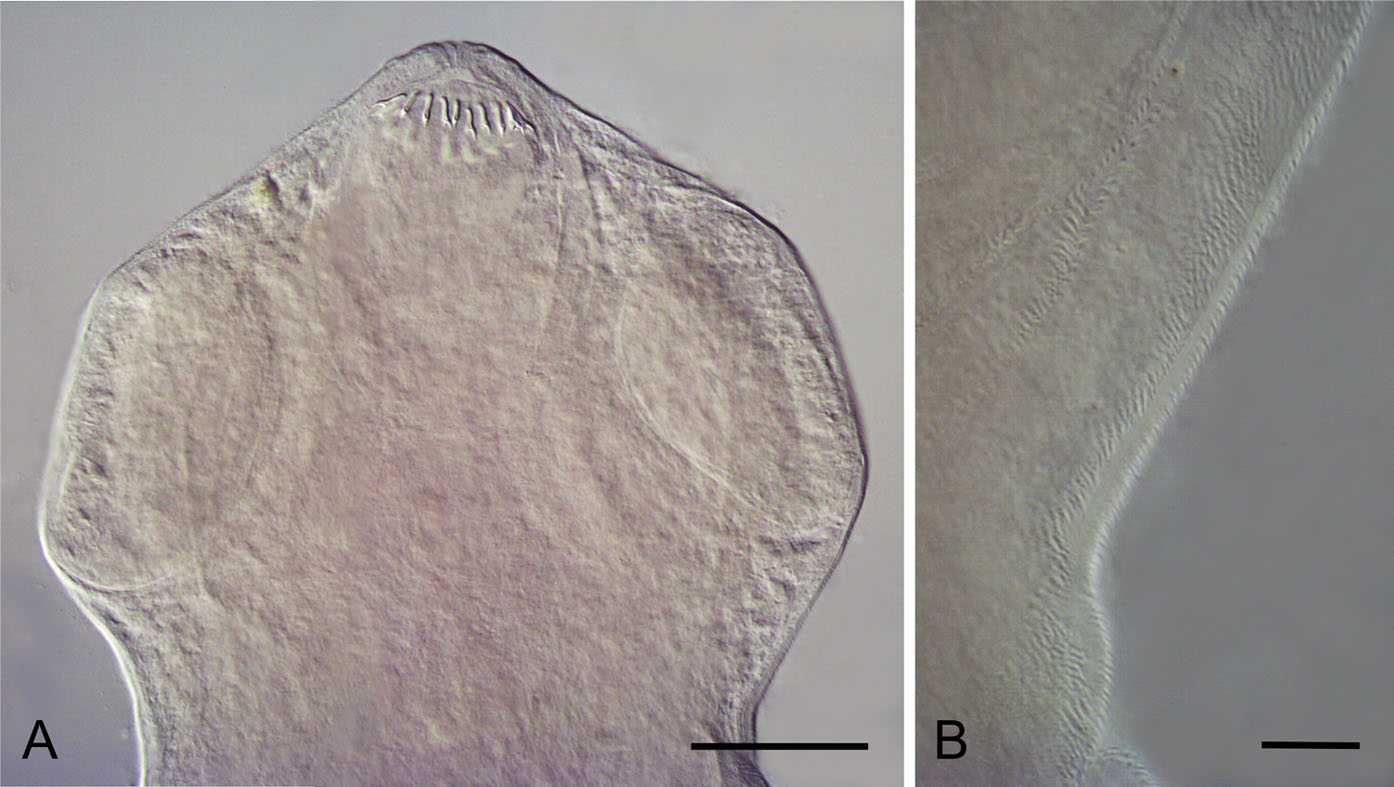
[Based on 3 fragmented specimens.] Body slender, ribbon-shaped, medium-sized, gradually widening posteriorly; maximum width at pregravid proglottides, 1510–1630 (1560, n = 5). Scolex oval, apically protruded; 111–158 (142, n = 3) long, with diameter 153–212 (177, n = 3) at level of posterior end of suckers ( Fig. 3A View Fig ). Scolex, neck and premature proglottides densely covered with large microtriches ( Fig. 6 View Fig ), becoming smaller with further maturation of strobila. Suckers round, muscular, with diameter 67–94 (79, n = 12). Rhynchus with short, conical apical protrusion anterior to crown of rostellar hooks when rostellum invaginated ( Figs. 3A View Fig , 6A View Fig ). Rostellum retractile, ovoid, thick-walled, with length 58–74 (65, n = 3) and maximum width at apical part 42–49 (46, n = 3), slightly tapering posteriorly; intensely stained glandular cells presented in its cavity. Rostellar sheath oval, thick-walled, not reaching level of posterior margins of suckers, 89–95 (92, n = 3) long, with maximum width 57–62 (59, n = 3) at its middle; intensely-stained glandular masses presented in its cavity ( Fig. 3A View Fig ). Rostellar hooks diorchoid, 18 (18, n = 3) in number, arranged in single row; each hook with long, slender handle with slightly curved end, sickleshaped blade, much shorter than handle, and welldeveloped guard ( Figs. 3B View Fig , 6A View Fig ). Measurements of hooks: total length 11–13 (12, n = 7); length of blade 3 (3, n = 7); length of base 8–9 (8, n = 7); distance between tip of blade and tip of handle 3–4 (3, n = 7). Neck slim, long, 99–126 (112, n = 3) wide, covered with distinct microtriches ( Fig. 6B View Fig ). Proglottisation beginning at 1.84–2.47 mm (2.16, n = 2) from posterior margins of suckers. Proglottides craspedote, much wider than long at all stages of development. Dorsal osmoregulatory canals narrow, with diameter 2–12 (5, n = 20). Ventral osmoregulatory canals 10–47 (30, n = 22) wide, with irregular transverse anastomoses with diameter 2–20 (8, n = 18); usually anastomoses situated along posterior proglottis margin, sometimes along transverse median line of proglottis ( Fig. 3 D View Fig ), passing ventral to testes, seminal receptacle and Mehlis’ gland, and dorsal to ovary, vitellarium and uterus ( Fig. 3C, D, F View Fig ). Sometimes, in neighbouring proglottides, 2 transverse anastomoses of ventral canals may join together forming short common duct near antiporal ventral osmoregulatory canal ( Fig. 3C, D View Fig ). Inner longitudinal muscle bundles of strobila numerous. Genital pores unilateral, sinistral, opening in anterior third of lateral proglottis margin. Genital atrium simple, thick-walled, surrounded by intensely stained cells, 27–54 (40, n = 13) deep; width diameter at base 10–32 (21, n = 14) and diameter at orifice 10–30 (17, n = 13) ( Fig. 5B, C View Fig ). Genital ducts dorsal to poral osmoregulatory canals.
Strobila protrandrous, with gradual maturation. Testes oval or with irregular shape, typically 3 (n = 131) in number per proglottis, rarely 4 (n = 16) and occasionally 5 (n = 1) per proglottis; testes with length 94–161 (123, n = 19) and width 62–94 (78, n = 19) in ‘‘male’’ mature proglottides ( Fig. 3E View Fig ). Position of testes may vary: usually 1 poral and 2 antiporal (n = 115) to female glands ( Figs. 3C, F View Fig ) or 2 poral and 1 antiporal (n = 14); rarely, all 3 testes arranged together antiporally (n = 2) to female glands ( Fig. 3D, E View Fig ); sometimes, in proglottides with more than 3 testes, they divided into 2 poral and 2 antiporal (n = 9), 1 poral and 3 antiporal (n = 6) or 3 poral and 1 antiporal (n = 1). Testes degenerating in postmature proglottides
before appearance of young uterus ( Fig. 4A View Fig ). External seminal vesicle along anterior margin of proglottis, dorsal to seminal receptacle, testes, ovary and vitellarium, tubular in young male proglottides ( Fig. 3E View Fig ), becoming voluminous, sac-like when filled of sperm, with maximum length 198–238 (221, n = 4) and width 50–89 (68, n = 4); connected to cirrus-sac by coiled canal with diameter 30–37 (34, n = 10). Cirrus-sac thin-walled, elongate, slightly narrowing at middle and with rounded antiporal end; passing along anterior proglottis margin, crossing osmoregulatory canals dorsally, never reaching midline of proglottis; maximum length 235–259 (244, n = 14) and width 45–82 (54, n = 14) in mature proglottides ( Figs. 3 View Fig , 5B View Fig ). Internal seminal vesicle oval or drop-shaped, 111–143 (131, n = 8) long and 37–57 (48, n = 8) wide, filling entire antiporal 1/3 to 1/2 of cirrus-sac ( Fig. 5B View Fig ). Ejaculatory duct 6–8 (6, n = 11) in diameter, coiled in cirrus-sac with invaginated cirrus; intensely-stained glandular cells surrounding internal walls of cirrus-sac and bottom of internal seminal vesicle. Evaginated cirrus cylindrical, 104–124 (110, n = 5) long; consisting of 3 distinct parts; basal part short, unarmed, 12–15 (15, n = 5) long, 22–25 (23, n = 5) wide; middle part 30– 37 X 22–25 (33 X 23, n = 5), densely covered with 19–23 (21, n = 10) rows of small triangular spines with maximum length 2–3 (3, n = 10); apical part unarmed, cylindrical, slightly tapering distally, with measurements 57– 87 X 12–20 (64 X 15, n = 5) ( Fig. 5D View Fig ).
Ovary median, ventral to testes and genital ducts, usually adjacent to anterior proglottis margin; almost fan-shaped, consisting of 5–8 (6, n = 17) compact oval lobes ( Fig. 3E View Fig ); fully-developed ovary 248–312 (281, n = 12) wide ( Fig. 4A View Fig ). Vitellarium with irregular shape, consisting of several compact oval lobes, median, posterior to ovary, 106–119 (112, n = 9) wide ( Figs. 3E View Fig , 4A View Fig ). Mehlis & gland almost compact, situated between vitellarium and ovary, dorsally to them ( Figs. 3F View Fig , 4A View Fig ). Seminal receptacle tubular when empty, becoming voluminous when filled of sperm, dorsal to female gonads and ventral to testes, cirrussac and external seminal vesicle ( Figs. 3E View Fig , 4A View Fig ); connecting with conductive part of vagina by tubular, thin-walled, coiled part, gradually enlarging and passing into voluminous sac-like part, 198–257 X 89 –109 (216 X 93, n = 7) ( Figs. 4A, B View Fig ). Vagina opening and passing ventral or postero-ventral to cirrus-sac; copulatory and conductive parts distinct ( Fig. 5C View Fig ). Copulatory part of vagina with cup-shaped poral portion, with length 35–46 (37, n = 6) and diameter at vaginal orifice 26–35 (28, n = 6), tapering and passing into narrow, tubular antiporal part 48–60 (52, n = 6) long and 16–19 (17, n = 6) wide; vaginal lumen covered by long, hair-like microtriches; genital atrium and copulatory vagina surrounded by thick sleeve of intensely-stained glandular cells ( Fig. 5C View Fig ). Conductive part of vagina thin, 5–8 (7, n = 8) wide, its lumen without microtriches, gradually passing into seminal receptacle at level of poral osmoregulatory canals ( Fig. 5C View Fig ).
Young uterus first appearing in fully-developed ‘‘female’’ mature proglottides as transversely-elongate, convoluted, thick-walled tube with slightlydeveloped labyrinthine structure and often with diverticula of poral end, situated in median field, dorsal to female gonads and ventral to cirrus-sac and genital ducts ( Fig. 4A View Fig ). Developing uterus transversely-elongate, thick-walled, with labyrinthine structure, forming deep diverticula and numerous sacculations ( Figs. 4B, C View Fig ). Gravid uterus thickwalled, sac-like, occupying entire median field, expanding above anterior margin of proglottis, never crossing lateral osmoregulatory canals ( Fig. 5A View Fig ). Ripe eggs oval; external shell thin, with diameter 40–55 (47, n = 9); embryophore thick, elliptical, 26– 32 X 18–25 (29 X 21, n = 13); oncospheres 20– 28 X 13–19 (23 X 17, n = 13); 3 pairs of embryonic hooks differing in length; median pair 12–13 (13, n = 10) long, internal lateral pair 10–11 (11, n = 11) long and external lateral pair with length 10–11 (10, n = 11) ( Fig. 5E View Fig ).
Remarks
According to the revision of the family Hymenolepididae by Czaplinski & Vaucher (1994), the avian hymenolepidid genera are divided into four subfamilies, i.e. Fimbriariinae Wolffhügel, 1899, Echinorhynchotaeniinae Mola, 1929, Hymenolepidinae Perrier, 1897 and Diploposthinae Poche, 1926. The presence of two pairs of osmoregulatory canals, with the genital ducts passing dorsally to them, unilateral genital pores, a rostellum armed with a single crown of hooks, a single set of male and female genitalia per proglottis and a small number of testes per proglottis indicate that the cestodes from Crithagra citrinelloides in Ethiopia belong to the subfamily Hymenolepidinae. This is the most specious hymenolepidid subfamily having more than 40 genera ( Czaplinski &
Vaucher, 1994; Mariaux et al., 2017). The majority of them are characterised by the presence of 8 or 10 rostellar hooks. The cestodes from Wondo Genet belong to a genus which is clearly distinguished from the remaining hymenolepidids of birds by a unique set of characters, i.e. it has 18 rostellar hooks, ventral osmoregulatory canals with transverse anastomoses, a variable number of testes per proglottis (mostly 3, rarely 4 or 5), and a unique sinistral position of the genital pores. In total, 11 avian hymenolepidid genera possess more than 10 hooks and have been compared with the cestodes of the present study ( Table 2). Five genera have 14 to 20 hooks, i.e. Hamatolepis Spasskii, 1962 (16 diorchoid hooks), Fuhrmannacathus Spasskii, 1966 (16 diorchoid hooks), Wardoides Spasskii, 1963 (14–18 coronuloid hooks), Oligorchis Fuhrmann, 1906 (14–16 arcuatoid hooks) and Hispaniolepis López-Neyra, 1942 (14–20 arcuatoid hooks). The specimens from Ethiopia differ from the abovementioned genera by the sinistral position of the genital pores ( Table 2). This character is unique among the avian Hymenolepididae where the unilateral dextral genital pores are a diagnostic character at the family level ( Czaplinski & Vaucher, 1994). Allohymenolepis Yamaguti, 1956 is the only genus with irregularly alternating pores ( Yamaguti, 1956). Another peculiar diagnostic character is the presence of transverse anastomoses of the osmoregulatory canals. Species of the majority of the avian hymenolepidid genera have no transverse anastomoses of the osmoregulatory canals, except for Fuhrmannacanthus spp., which has transverse anastomoses of the ventral canals passing along the posterior margins of the proglottis ( Czaplinski & Vaucher, 1986). In addition, the cestodes from Ethiopia differ from the species of Fuhrmannacanthus by the number of testes (10–20) and by the presence of an accessory sac. Additional differences between the cestodes from Ethiopia and the avian hymenolepidids with a range of 10–20 rostellar hooks are: (i) the shape of hooks, i.e. arcuatoid in Oligorchis and Hispaniolepis ; (ii) the constant number of testes, i.e. 3 in Hamatolepis , Hispaniolepis and Wardoides , and 4 in Oligorchis ; (iii) the different group of the definitive hosts, i.e. Anseriformes, assuming aquatic life-cycles in Wardoides , Fuhrmannacanthus and Hamatolepis (see Spasskaya, 1966).
Six avian hymenolepidid genera are characterised by more than 20 rostellar hooks and by this character differ from the specimens from Ethiopia ( Table 2). This group includes four genera with species parasitic in terrestrial birds ( Thaumasiolepis Mariaux & Vaucher, 1989 , Monorcholepis Oshmarin, 1961 , Paradicranotaenia López-Neyra, 1943 and Ortleppolepis Spasskii, 1965 ), and two genera with species parasitic in Anseriformes ( Dicranotaenia Railliet, 1892 and Nematoparataenia Maplestone & Southwell, 1922 ) ( Table 2). Two of these, Thaumasiolepis and Ortleppolepis , both monotypic, were reported from terrestrial birds in Africa. Thaumasiolepis microarmata Mariaux & Vaucher, 1989 is a parasite of Piciformes; it differs from the cestodes from Ethiopia by the spiniform shape of the rostellar hooks, the presence of a small rounded rostellum, dextral genital pores, three testes in a triangular configuration and the genital ducts passing between the dorsal and ventral poral osmoregulatory canals ( Mariaux & Vaucher, 1989). The genus Ortleppolepis , with the type- and only species O. multiuncinata ( Ortlepp, 1963) Spasskii, 1965 , has been described from Guttera edouardi (Hartlaub) (Galliformes) in Central Africa. It differs from the species from Wondo Genet by the presence of 70 hammer-like hooks arranged in five rows ( Ortlepp, 1963).
The presence of more than ten rostellar hooks and the variable number (> 3) and position of the testes are more frequently found in hymenolepidids from mammals ( Czaplinski & Vaucher, 1994). Therefore, we additionally compared the cestodes from Wondo Genet with 16 hymenolepidid genera from mammals which have more than ten rostellar hooks ( Table 2). Fourteen genera are characterised by unilateral dextral genital pores, which clearly distinguish them from our material from Ethiopia. Some of the genera could be also distinguished by the presence of a hyperapolitic strobila ( Pseudhymenolepis Joyeux & Baer, 1935 ); the presence of a bifurcate guard of the rostellar hook ( Triodontolepis Yamaguti, 1959 ); the presence of an invaginable rostellum ( Relictolepis Gulyaev & Makarikov, 2007 and Staphylocystoides Yamaguti, 1952 versus the retractile rostellum of C. citrili n. sp.); the gravid uterus extending beyond the osmoregulatory canals in Rodentolepis Spasskii, 1954 and Staphylocystis Villot, 1877; and the presence of polar filaments of embryophores in Nomadolepis Makarikov, Gulyaev & Krivopalov, 2010 (see Spasskii, 1954; Czaplinski & Vaucher, 1994; Gulyaev & Makarikov, 2007; Makarikov et al., 2010; Greiman et al., 2013; Tkach et al., 2013).
Two hymenolepidid genera from mammals are characterised by the sinistral position of the genital pores, i.e. Pararodentolepis Makarikov & Gulyaev, 2009 , a genus with species parasitic in rodents and insectivores ( Makarikov & Gulyaev, 2009; Greiman & Tkach, 2012) and Sawadalepis Makarikova & Makarikov, 2013 with species parasitic in bats ( Makarikova & Makarikov, 2013). The two species of Pararodentolepis , P. sinistra Makarikov & Gulyaev, 2009 and P. gnoskei ( Greiman & Tkach, 2012) , resemble the specimens from Ethiopia in having testes in a line, separated by the female gonads, a distinct copulatory part of the vagina, a saclike uterus not extending beyond the osmoregulatory canals. However, they have three testes, situated in a constant position, i.e. one poral and two antiporal to the female gonads, the osmoregulatory canals are without transverse anastomoses, the uterus is bi-lobed and the embryophores have hair-like polar filaments ( Makarikov & Gulyaev, 2009; Greiman & Tkach, 2012). The genus Sawadalepis , with type-species S. prima Makarikova & Makarikov, 2013 , differs from our specimens by the peculiar bilaterally shifted position of the osmoregulatory canals. The three testes are arranged in a compact line, not separated by female gonads, and the uterus extends beyond the osmoregulatory canals into lateral fields of the proglottis ( Makarikova & Makarikov, 2013). In addition to these two genera with a sinistral position of the genital pores, the genus Paramilina Makarikova, Gulyaev, Tiunov & Feng, 2010 (parasitic in bats) has a variable position of the genital pores, i.e. different parts of the strobila are characterised by dextral or sinistral pores. It differs from our specimens from Ethiopia by the structure of the scolex, having a rudimentary rostellum without armament ( Makarikova et al., 2010).
On the basis of this differential diagnosis, we considered the specimens from Crithagra citrinelloides from Ethiopia as belonging to a new genus, Citrilolepis n. g., with type-species C. citrili n. sp.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |