Plesiothoa gerroa, Gordon, 2020
Gordon, Dennis P., 2020, New Hippothoidae (Bryozoa) from Australasia, Zootaxa 4750 (4), pp. 451-476 : 456-459
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4750.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:AE9FDD46-5471-44B3-97FB-11C4BD45C59B |
|
DOI |
https://doi.org/10.5281/zenodo.3717932 |
|
persistent identifier |
https://treatment.plazi.org/id/9DD7D35C-A910-4755-B784-78F64EB16EE8 |
|
taxon LSID |
lsid:zoobank.org:act:9DD7D35C-A910-4755-B784-78F64EB16EE8 |
|
treatment provided by |
Plazi |
|
scientific name |
Plesiothoa gerroa |
| status |
sp. nov. |
Plesiothoa gerroa n. sp.
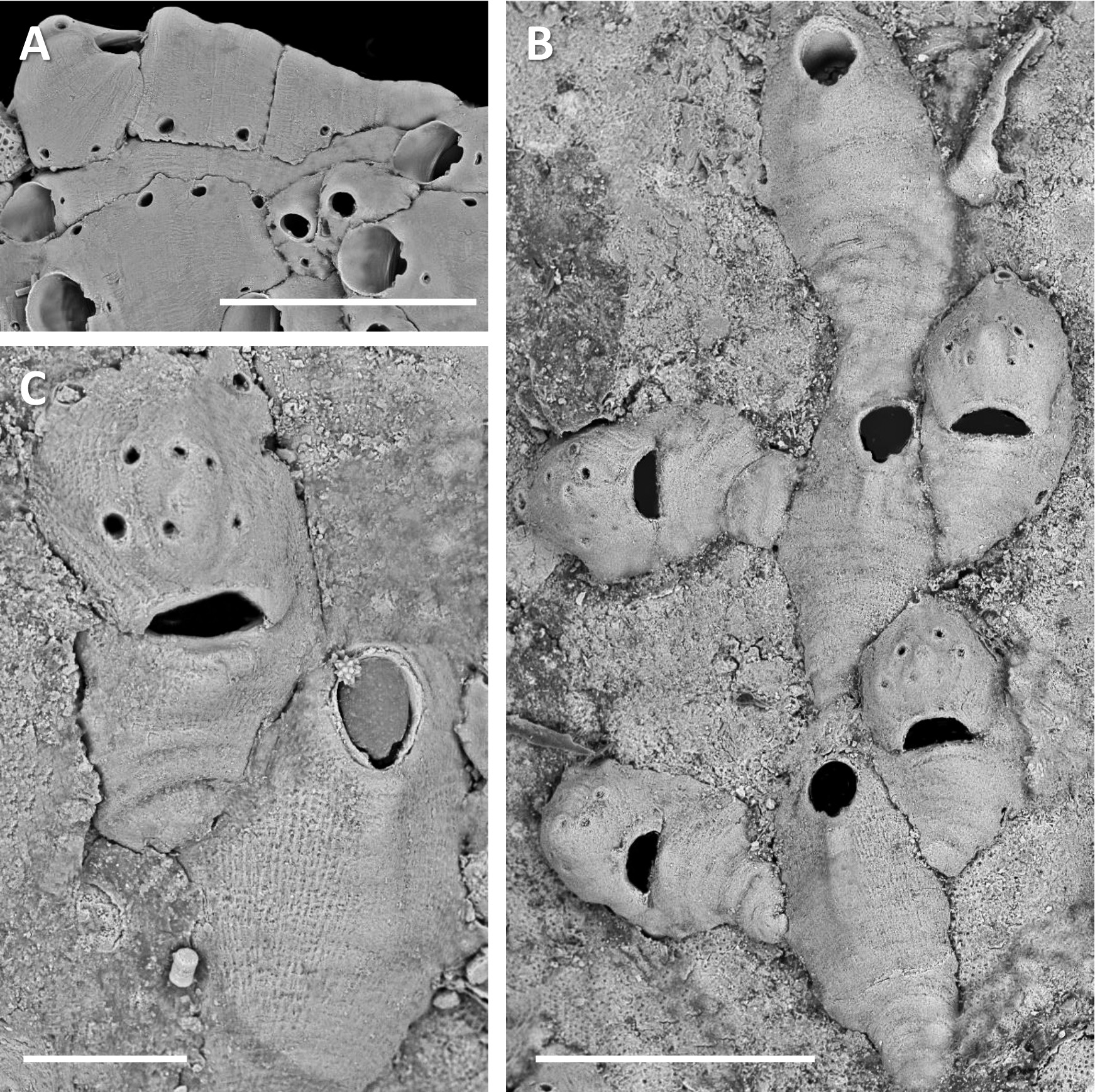
( Figs 3C View FIGURE 3 ; 4 View FIGURE 4 A–E, 5A)
Etymology. From Gerroa, a community on the New South Wales coast; place name used in apposition.
Material examined. Holotype: Australian Museum U.5790, from 34.7814° S, 150.8228° E, on the coralline red alga Amphiroa anceps (Lamarck) Decaisne submerged in midtidal pools, Black Head, Gerroa, NSW. Paratype: Australian Museum U. 5791, same data as for holotype . Other material (non-type): NIWA 132856 View Materials , same data as for holotype .
Description. Colony small, confined to algal internodes (intergenicula) hence varying through linear, uniserial to multiserial phases, and unilaminar to multilamellar ( Figs 4A, B View FIGURE 4 ), attaining 6–7 mm length but boundaries of abutting colonies can be difficult to determine at a macro level.
Autozooids in early and uncrowded later parts of colony bilaterally symmetrical and clavate with caudae of varied length, sometimes very long ( Fig. 3C View FIGURE 3 ), especially in axial runners; autozooids budded laterally have very short to non-existent caudae. Autozooidal shape in crowded parts of colony very variable depending on available space. Gymnocystal shield smooth-surfaced or with fine striae, gently elevated toward weak suboral umbo; occasional transverse growth lines in shield. Lateral margins of zooid smooth to variably crenulate. ZL 436±130, 248–868 (26), ZW 209±24, 155–274 (26).
Orifice inclined downwards distad, a little longer than wide viewed en face; anter widest midlength, narrowing proximad toward stout squared condyles set below short shoulders of orifice; sinus U-shaped with essentially paral- lel sides ( Fig. 4C View FIGURE 4 ); generally a tiny pseudopore in weak suboral umbo. OL 81±8, 73–107 (13), OW 73±4, 66–79 (13).
Internal distal wall of autozooid a broad inverse V as seen in transparency, with tiny communication pore on either side. Lateral autozooidal communications typically 2–4 along each wall, comprising small, rounded-triangular pore-chambers in base of wall. Budding can occur from all or none of the pore-chambers in open parts of colony but all appear to be involved in interzooidal communication in dense parts of colony where zooids overlap. The laterodistal pair can give rise to 0–2 autozooids, female zooids or zooeciules. Female zooids are also typically budded from the distolateral pair. In crowded parts of colony where almost no substratum space is visible, adventitious budding can occur and zooid orientations are more varied ( Fig. 4B View FIGURE 4 ). Abundant interzooidal communication occurs in crowded colony parts among all the zooid morphs.
Female cystids frequently broader (especially in distal third) than parent autozooids, narrowing somewhat proximally but non-caudate. Ovicell prominent, terminal, cleithral; ooecium formed by distal kenozooid; ooecial surface smooth, typically with small pair of apical pseudopores or these fused; lateral profile not higher than zooidal frontal shield ( Fig. 5A View FIGURE 5 ). Dimorphic combined maternal aperture similar in size to that of autozooids, but broader. Ooecial kenozooid with distal pore-chamber opening. Lateral pore-chamber openings of female zooid as in autozooids. ♀ ZL 420±63, 307–533 (13); ♀ ZW 214±28, 165–253 (13); OoL 165±12, 145–182 (13); OoW 195±20, 161–224 (13); ♀ OrL 70±3, 65–74 (13); ♀ OrW 76±5, 66–85 (13).
Zooeciules typically squat structures on laterodistal shoulders of autozooids and female zooids, with rounded orifice bearing a pair of proximolateral condyles and broad sinus ( Fig. 4C View FIGURE 4 ). Operculum present. ZoL 123±35, 81–201 (13); ZoW 122±23, 85–153 (13); ZoOL 31±3, 24–34 (10); ZoOW 32±4, 26–35 (10).
Ancestrula longitudinally oval, margin conspicuously crenulated ( Fig. 4E View FIGURE 4 ); resembling autozooids but without cauda and no apparent lateral pore-chambers. Daughter autozooid mid-distal; later astogenetic stages not seen. AncL 237±19, 224–250 (2); AncW 203±7, 198–208 (2).
Remarks. Plesiothoa gerroa n. sp. has almost as much within-colony variability as P. densa n. sp., but its autozooids are never quite as Hippothoa -like in early axial runners. The zooeciules closely resemble those in P. trigemma but their orifices are not reversed as in P. trigemma ; rather they tend to be orientated transversely, relative to the parent autozooid or female zooid. The ancestrula has more crenulations than does P. trigemma but both have a subperipheral band of gymnocystal pitting. Plesiothoa gerroa n. sp. resembles the barnacle-encrusting species P. coquimbana , but the latter lacks zooeciules and its ancestrula is not crenulated. In New Zealand, Plesiothoa australis Moyano & Gordon, 1980 occurs on the geniculate coralline alga Corallina officinalis Linnaeus , but autozooids are easily distinguished by the scoop-like autozooidal orifice with marginal lappets.
Detached zooids provided the opportunity to examine shield interiors, revealing that zooids at the colony margin have variable spinules on the underside of the proximal frontal shield ( Fig. 4D View FIGURE 4 ). These are temporary, being resorbed and absent from later zooids.
Distribution. Endemic; known only from the type locality, Black Head, Gerroa, New South Wales, Australia, in mid–low-tidal rock pools on Amphiroa anceps . Given the small colony size, transparency and tendency to occur low on the algal branches, it can be easily overlooked. Nevertheless, given the commonness of the algal host, the bryozoan is likely to be fairly widely distributed along the temperate Australian coastline.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |