Delgadobius amazonensis Chani-Posse & Couturier, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.211551 |
|
publication LSID |
lsid:zoobank.org:pub:F74F62A5-4398-4D55-B5AE-751020A3CFFA |
|
DOI |
https://doi.org/10.5281/zenodo.6172335 |
|
persistent identifier |
https://treatment.plazi.org/id/601CA77A-FF95-FF98-BFD7-0B1B7BA5FE8F |
|
treatment provided by |
Plazi |
|
scientific name |
Delgadobius amazonensis Chani-Posse & Couturier |
| status |
sp. nov. |
Delgadobius amazonensis Chani-Posse & Couturier View in CoL , new species
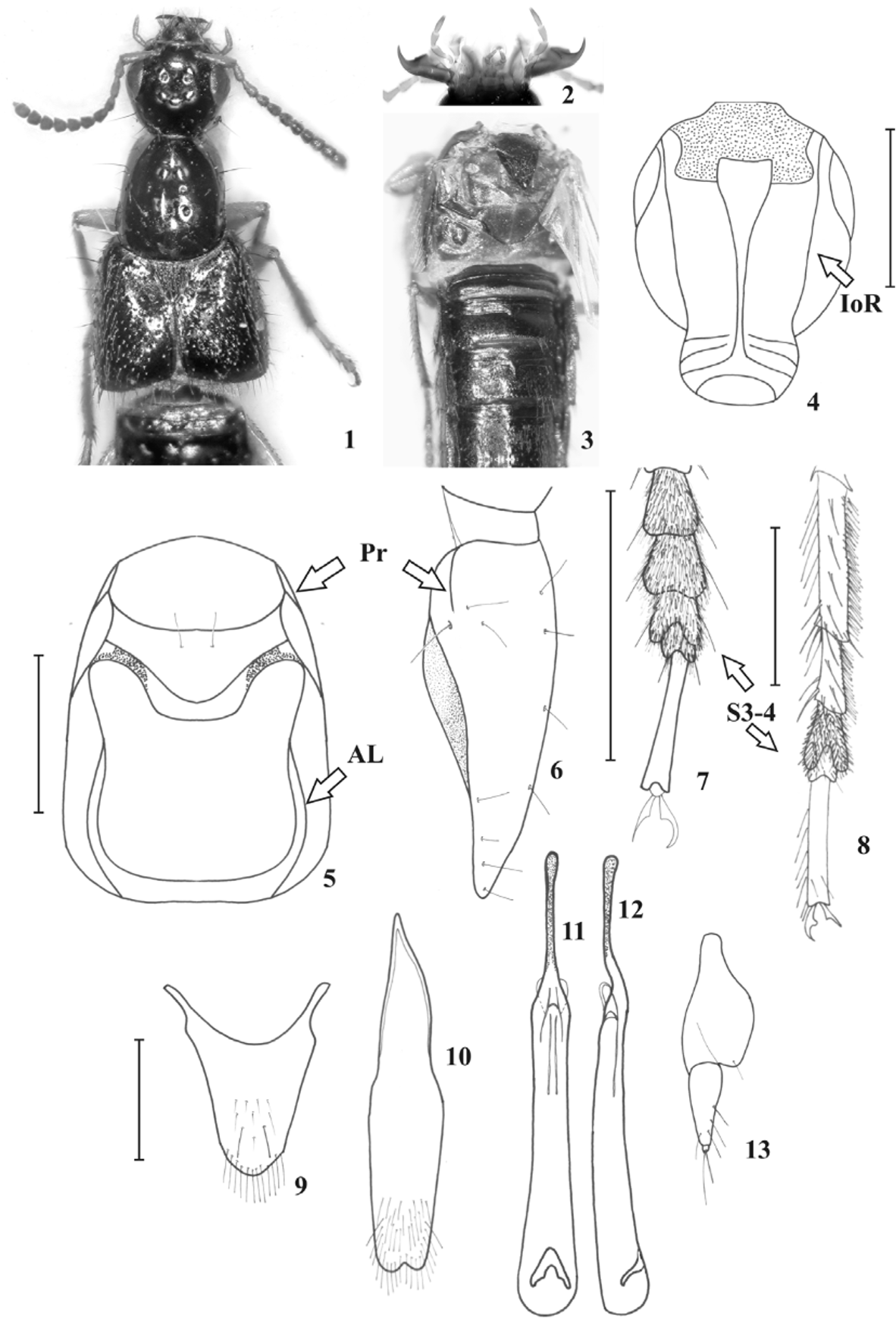
( Figs. 1–13 View FIGURES 1 – 13 )
Diagnosis. As for the genus (see above).
Description. Length of the body 7.5–7.9 mm (3.8–4.2 mm, abdomen excluded). Coloration as for the genus.
Head of rounded-quadrangular shape, slightly broadened at distal third and with obtuse hind angles, as long as to slightly wider than long (HW/HL= 1.00–1.11) ( Fig. 1 View FIGURES 1 – 13 ), slightly narrower than pronotum at widest point (HW/ PW= 0.88–0.91); postmandibular ridge present; epicranium with medial interocular punctures separated by a distance less than 1.5 times as large as distance separating medial punctures from lateral punctures. Eyes distinctly longer than temples seen from above (EL/TL= 1.75) ( Fig. 1 View FIGURES 1 – 13 ). Antennae with segment 1 shorter than segments 2 and 3 combined, segment 3 slightly longer than segment 2, segments 4 and 5 longer than wide, segment 6 about as long as wide, segments 7 to 10 transverse, last segment minutely emarginate. Mandibles (right and left) with a single tooth ( Fig. 2 View FIGURES 1 – 13 ). Maxillary palpus with last segment gradually narrowed to subacute apex from apical half, about 1.5 times as long as preceding segment and not appreciably narrower ( Fig. 2 View FIGURES 1 – 13 ). Mentum and submentum subequal in length. Labial palpus with segment 3 distinctly longer than segment 2 and gradually narrowed to subacute apex ( Fig. 2 View FIGURES 1 – 13 ).
Pronotum slightly longer than wide (PW/PL= 0.89–0.95) ( Fig. 1 View FIGURES 1 – 13 ), slightly narrowed anteriad; dorsal surface of pronotum with two rows of punctures, each with four punctures. Legs with front and middle tarsi as long as front and middle tibiae, hind tarsi shorter than hind tibiae; front tarsus with first segment shorter than segments 2 and 3 combined ( Fig. 7 View FIGURES 1 – 13 ); mid and hind tarsus with first segment longer than segments 2 and 3 combined ( Fig. 8 View FIGURES 1 – 13 ); first segment of hind tarsus longer than last segment (S1/S5= 1.3–1.5).
Elytra at sides distinctly longer than pronotum at midline (EtL/PL=1.2–1.3).
Abdomen. Terga 3 to 5 with posterior transverse basal carina, acutely extended medially on terga 3 and 4, incomplete on tergum 5; terga 6 to 8 with only anterior transverse basal carina.
Male genitalia. Sternum 7 slightly emarginate medio-apically; sternum 8 deeply emarginate medio-apically; tergum 10 subangulate at apex, with several apical setae and two to four long and strong subapical setae ( Fig. 9 View FIGURES 1 – 13 ); sternum 9 acutely emarginate apically, with several apical setae at each side of emargination and one to two long subapical macrosetae ( Fig. 10 View FIGURES 1 – 13 ). Aedeagus with parameres fused as one short sclerite only attached to median lobe at base, without sensory peg setae; median lobe elongate, with apical part distinctly narrowed into a thin rod-like apex; internal sac with sclerotized structures ( Figs. 11, 12 View FIGURES 1 – 13 ).
Female genitalia. Sterna 7 and 8 straight to slightly sinuate apically. Genital segment with styli of tergum 9 similar to those of male; tergum 10 similar to that of male; gonocoxites strongly developed, second gonocoxites rather short, each with two to five strong setae along its outer margin, with a minute stylus ( Fig. 13 View FIGURES 1 – 13 ) and two long apical setae.
Etymology. The name refers to the species distribution.
Type material. Holotype 3, with labels: “ Peru – Loreto – 30.X.2010 / Iquitos Nauta road, km 46/ 04° 02´S 73° 24´W / G. Couturier, C. Delgado col.”, “on inflorescence 3 in anthesis of Mauritia flexuosa Arecaceae ”, “ Holotype Delgadobius amazonensis Chani-Posse & Couturier 2012 ” (red label) ( MNHN). Twenty-four paratypes with same data, 1 3 3 Ƥ ( FMNH), 2 3 3 Ƥ ( IADIZA), 1 3 5 Ƥ ( MNHN), 2 3 3 Ƥ ( UNALM), 1 3 3 Ƥ ( UTCI). Other thirtyseven paratypes, with labels: “ Peru – Loreto – 16.X.2008 / Pucaurquillo (Pevas)/ 03°20´S, 72°04´W / G. Couturier, W. Gonzáles col.,“on inflorescence Euterpe precatoria Arecaceae (beginning anthesis)”, “ Paratype Delgadobius amazonensis Chani-Posse & Couturier 2012 ”, 2 3 1 Ƥ ( IADIZA), 1 3 1 Ƥ ( MNHN), 1 3 1 Ƥ ( MNHUB), 1 3 1 Ƥ ( NMW); “ Peru Loreto Iquitos/ Carretera Iquitos Nauta km/ 31.X.1991 /K. Mejia Col.”, “Host plant Mauritia carana ”, “ Paratype Delgadobius amazonensis Chani-Posse & Couturier 2012 ”, 1 3 1 Ƥ ( MNHN); “ PEROU / Iquitos/ Sept. 1985 / F. Kahn Réc.”, “sur "Huassai"/ Euterpe precatoria ”, “ Paratype Delgadobius amazonensis Chani-Posse & Couturier 2012 ”, 1 3 ( MNHN), 1 3 1 Ƥ ( ZMUC); “ PEROU / Iquitos/ Sept. 1985 / F. Kahn Réc.”, “sur "Ungurahui""/ Jessenia bataua ”, “ Paratype Delgadobius amazonensis Chani-Posse & Couturier 2012 ”, 3 3 2 Ƥ ( IADIZA), 2 3 2 Ƥ ( MNHN), 2 3 1 Ƥ ( MNHUB), 2 3 1 Ƥ ( NMW), 2 3 1 Ƥ ( UNALM);“ Brésil – Amaz./ Manaus/ 19.V.1996 / G. Couturier & F. Kahn”, “Km. 130/ Br134/ Rodovia Boa Vista”, “ Mauritia flexuosa Palmae sur inflorescence mâle”, “ Paratype Delgadobius amazonensis Chani-Posse & Couturier 2012 ” 1 3, ( IADIZA), 3 3, ( INPA), 1 3, ( MNHN).
Distribution and habitat. Delgadobius amazonensis has been recorded from Peru (Iquitos) and Brazil (Manaus). It was found in association with palm trees ( Arecaceae ) during anthesis, on male inflorescences of Mauritia flexuosa L. f., Mauritia carana Wallace , Oenocarpus bataua Martius (= Jessenia bataua ) and Euterpe precatoria Martius.
The occurrence of the new species in Mauritia flexuosa was reported from two localities in Peru (Iquitos) and Brazil (Manaus). Given this association, it seems probable that both the insect and the host plant may share a common distribution. Mauritia flexuosa is an Amazonian native palm species ( Figs. 14–21 View FIGURES 14 – 21 ), that is distributed all over the American tropics and the East Andes, mainly in the Amazonian basin, in areas such as Peru, Bolivia, Colombia, Ecuador, Venezuela, Brazil, the Guyanas, north of Trinidad and Panama ( Henderson et al., 1995). This palm species plays a major role in the economic, social and ecological systems of the Peruvian Amazonia ( Vásquez et al., 2008, Trujillo-González et al., 2011). Because of its importance and in order to avoid its overexploitation, this plant is in process of domestication to be cultivated as a dwarf form ( Vásquez et al., 2008). However, the development of the crops involves the proliferation of harmful insects and knowledge about potential natural enemies is required. Mauritia flexuosa grows naturally on flooded soils, on rivers and stream borders forming dense monospecific gatherings known in Peru as “aguajales” ( Figs. 14, 15 View FIGURES 14 – 21 ). When they are associated with other palm species such as Oenocarpus bataua (as it is also the case here for the occurrence of Delgadobius ), they are called “sacha-aguajales” ( Delgado et al., 2007). In M. flexuosa , the new species has been observed actively running among the rachillae ( Figs. 17–19 View FIGURES 14 – 21 ) of the palm together with adults and larvae of Mystrops dalmasi Grouvelle ( Coleoptera : Nitidulidae ), which is quite abundant (Couturier, pers. obs.) and may be potential prey. Nitidulidae has also been reported as the most abundant family visiting male inflorescences of M. flexuosa among Coleoptera , which in turn has shown the highest representation among the insect orders associated with this palm species in Manaus, Brazil ( Storti, 1993). The presence of D. amazonensis in M. flexuosa has only been observed during the flowering event, at the end of which the insect disappears (G. Couturier, pers. obs.). The host plant, Mauritia flexuosa or “aguaje” ( Fig. 14, 15 View FIGURES 14 – 21 ) as it is called in the Peruvian Amazonia (“buriti” or “miriti” in Brazil) starts its commercial fructification at the age of 12–15 years when it naturally grows. Given its height (35–40 m), it has been a common practice to cut the whole female individual in spite of the detrimental effects on the palm-tree populations (G. Couturier, pers. com.). The dwarf form or “aguaje enano” ( Fig. 14 View FIGURES 14 – 21 ) has not been found in natural conditions but only in culture. Its fructification happens at the age of 5 years, before the stem is less than 1 m high. The precocity of its fructification and its short stem allows easy collection of both the fruit and the flower-visiting insects ( Fig. 18, 21 View FIGURES 14 – 21 ). Flowering can occur during the whole year. The anthesis of female and male flowers usually begins at 16 h and lasts an average of 24 h. When the flowers open, they produce a fragrance that may act as a mechanism for attracting insects ( Delgado et al., 2007). A great amount of insects associated with this palm has been reported from male and female inflorescences during anthesis ( Storti, 1993). Since M. flexuosa is a dioecious palm, pollination by insects may be crucial.
A common feature among the genera of palm trees from which D. amazonensis has been recorded ( Mauritia , Euterpe and Oenocarpus ) is the rather sparse structure of their inflorescence compared to other genera of Arecaceae , such as Astrocaryum , Elaeis and Phytelephas . These genera have been also examined in the field but D. amazonensis was not either observed or reported. We assume that the structure of the inflorescence may be a limiting factor in the predatory behavior of D. amazonensis . Because of its association with a dominant plant species of economic and cultural importance in Amazonia, the ecological role of D. amazonensis in the “aguajal” ecosystem may deserve further studies of applied interest in the future.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |