Archboldomys luzonensis Musser, 1982
|
publication ID |
https://doi.org/ 10.1206/3754.2 |
|
persistent identifier |
https://treatment.plazi.org/id/564087D2-FFDA-FFF6-FE4E-FD4935466D0D |
|
treatment provided by |
Carolina |
|
scientific name |
Archboldomys luzonensis Musser, 1982 |
| status |
|
Archboldomys luzonensis Musser, 1982 View in CoL
HOLOTYPE: FMNH 95122 About FMNH . Young adult male collected on 24 April 1961, field number D.S. Rabor 1149 ( Musser, 1982a: figs. 13, 22–32). Prepared as stuffed skin with skull (fig. 10A) removed and cleaned.
TYPE LOCALITY: Philippines: Luzon Island: Camarines Sur Province: Mt. Isarog , 6560 feet .
MEASUREMENTS: Tables 1 and 2.
SPECIMENS EXAMINED (N = 14): Camarines Sur Province, Mt. Isarog National Park , 1350– 1750 m ( FMNH 147172 About FMNH , 147173 About FMNH , USNM 573505 About USNM , 573834–573840 About USNM , PNM 4683 View Materials ); 4 km N, 21.5 km E Naga City, 1550 m ( FMNH 152031 About FMNH , 152032 About FMNH ) ; Pili Municipality, Mt. Isarog , 6560 ft ( FMNH 95122 About FMNH [holotype]) .
EMENDED DIAGNOSIS: A shrew mouse of the genus Archboldomys , readily distinguished from A. maximus , n. sp., by the following combination of external, cranial, and dental features: (1) smaller body; (2) shorter, reddish-brown pelage; (3) absolutely and relatively shorter tail, with fewer scale rows (TSR); (4) absolutely and relatively shorter hind foot; (5) shorter and narrower skull; (6) longer incisive foramina; (7) larger (though short and broad) interpremaxillary foramen; (8) wider zygomatic plate; (9) greater orbito-temporal length; (10) shorter and narrower pterygoid ridge, terminating posteriad below the alisphenoid strut; (11) broader and conspicuously exposed alisphenoid strut (see Musser, 1982a: fig. 23A); (12) slightly more elongate and narrowly angled posterior edge of upper incisors; (13) narrower and shorter maxillary and mandibular molar rows; (14) smaller molars in which cusp t3 and t9 are either missing from M1 or so broadly merged with the adjacent cusp in the respective cusp row that they are unidentifiable; (15) shorter and broader mandible; (16) broader angular process; and (17) longer and more robust coronoid process (figs. 10A, 13; table 11).
KARYOLOGY: 2N = 26 and FN = 43 (fig. 6A; Rickart and Musser, 1993; Rickart and Heaney, 2002).
COMPARISONS: See Description and Comparisons section of A. maximus , below.
DISTRIBUTION: Known only from Mt. Isarog, southeastern Luzon Island ( Rickart et al., 1991; Balete and Heaney, 1997; Heaney et al., 1999).
ECOLOGY: Archboldomys luzonensis is a montane and mossy forest specialist, occurring at 1350 m to 1750 m; it was not recorded below 1350 m ( Rickart et al., 1991; Balete and Heaney, 1997; Heaney et al., 1999). Laurels, myrtles, oaks, and podocarps were the common canopy and emergent trees in these forests, often reaching a canopy height of ca. 12–20 m in montane forest, and 5–12 m in mossy forest. Moss cover ranged from moderate in montane forest, to covering most surfaces of trees and ground in mossy forest; leaf litter in both habitats was extensive, and the humus layer was thick. Vegetation of these habitats was described in more detail in Heaney et al. (1999).
The morphological adaptations of A. luzonensis (e.g., small, robust body with short tail, short limbs, and small ears, short dense pelage, and long, sharp claws) suggest a semifossorial habit. It is active during the day, possibly extending from dawn to dusk; in 1988, seven of eight individuals captured were taken during the day and the eighth near dawn ( Heaney et al., 1999). They were captured mostly along runways under root tangles, under fallen logs, or beside ground vegetation. In 1993–1994, two individuals were observed foraging in leaf litter in mossy forest during the day (Balete and Heaney, 1997). Stomach contents, consisting of amphipods, larval and adult arthropods, and earthworms, suggest a vermivorous/insectivorous diet ( Heaney et al., 1999). Earthworms were abundant where this species was captured ( Rickart et al., 1991). Females have two pairs of inguinal mammae. Two pregnant females, recorded in late March and late April, each had a single embryo ( Heaney et al., 1999). The species appeared to be moderately common in mossy forest on Mt. Isarog, with an estimated density of ca. 4.5 + 1.63 individuals/ha (Balete and Heaney, 1997).
Other nonvolant small mammals recorded along with Archboldomys luzonensis in montane and mossy forest included Crocidura grayi , Apomys microdon , A. musculus , Batomys cf. granti , Chrotomys gonzalesi , Phloeomys cumingi , Rattus everetti , and Rhynchomys isarogensis (Balete and Heaney, 1997; Heaney et al., 1999; Rickart et al., 1991). Of these, five are members of the Chrotomys Division: A. microdon , A. musculus , C. gonzalesi , and R. isarogensis (table 12).
Archboldomys maximus , new species
Figures 8B View FIG , 11A, 12, 13
HOLOTYPE: FMNH 193531 About FMNH . Adult male collected on 23 March 2007, field number D.S. Balete 4560. Fresh tissues were removed from the thigh and placed in 95% ethanol. The rest of the specimen was initially fixed in formalin, now preserved in ethyl alcohol with skull (fig. 13) removed and cleaned. The holotype is currently housed at FMNH but will be transferred to PNM.
TYPE LOCALITY: Philippines: Luzon Island: Mountain Province: Barlig Municipality : 1.75 km N, 0.4 km W Mt. Amuyao peak, 1885 m elevation, 17.02929° N, 121.12466° E (fig. 1) GoogleMaps .
MEASUREMENTS: Tables 1 and 2.
SPECIMENS EXAMINED (N = 13): Mountain Province, Barlig Municipality, Mt. Amuyao peak, 2690 m ( FMNH 193524 About FMNH ); 0.5 km N, 0.5 km W Mt. Amuyao peak, 2530 m ( FMNH 193522 About FMNH , 193523 About FMNH ); 0.4 km N, 0.4 km W Mt. Amuyao peak, 2480 m ( FMNH 193525–193527 About FMNH ); 0.75 km W Mt. Amuyao peak, 2300 m ( FMNH 193528 About FMNH , 193942 About FMNH , 193943 About FMNH ); 1.0 km N, 1.0 km W Mt. Amuyao peak, 2150 m ( FMNH 193529 About FMNH , 193530 About FMNH ); 1.75 km N, 0.4 km W Mt. Amuyao peak, 1885 m (193531 [holotype], 193944).
ETYMOLOGY: From the superlative of magnus (Latin: “great, large”), to highlight its being the largest of the seven species of shrew mice ( Archboldomys , Crunomys , and Soricomys ) on Luzon. We recommend as the English common name either “large Cordillera shrew mouse” or “Cordillera archboldomys.”
DIAGNOSIS: Archboldomys maximus is a shrew mouse similar to A. luzonensis , but readily defined by the following combination of external, cranial, and dental characters and proportions (tables 1, 2, figs. 9–14): (1) long, robust body; (2) dark chestnut pelage; (3) tail nearly as long as head and body, with fine scale rows; (4) relatively long hind feet; (5) long, broadly ovate skull with tapered rostrum and more strongly inflated cranium; (6) proportionately short incisive foramina; (7) interpremaxillary foramen small and slightly elongate, visibly posterior to line connecting the posterior edges of incisors (fig. 9); (8) narrow zygomatic plate with pronounced slanting anterior edge; (9) short orbito-temporal length; (10) long and wide pterygoid ridge extending posteriad to become the pterygoid bridge over the foramen ovale; (11) narrow and delicate alisphenoid strut; (12) narrow, sharply angled posterior edge of upper incisors (fig. 9); (13) long molariform tooth row; (14) large molars with complete cuspidation on the first molar (cusps t3 and t9 clearly present but broadly merged with adjacent cusps; cusp t8 with anterolingual projection); (15) long and narrow mandible; (16) narrow angular process; and (17) short and narrow coronoid process.
DESCRIPTION AND COMPARISONS: Archboldomys maximus is the larger of the two species of Archboldomys , and is readily distinguishable externally from A. luzonensis by its longer and more robust body overall (smaller, shorter body in A. luzonensis ); dark grayish chestnut pelage (reddish brown); absolutely and relatively longer tail, with finer scale rows (absolutely and relatively shorter tail, with larger and fewer scale rows); longer, broader, and more darkly pigmented hind feet (shorter, narrower, and paler hind feet) (fig. 12, table 1; Musser, 1982a: fig. 22).
The pelage of A. maximus is uniformly grayish chestnut, longer and thicker dorsally than ventrally. The rostrum is tapered and the face is broad (fig. 12A). The pale grayish-brown vibrissae extend slightly beyond the ears. The lips and rhinarium are pigmented pale grayish brown. The eyelids are finely edged with dark gray, surrounded by a narrow paler band covered with short dark grayish-brown fur. The ears are small, round, medium grayish brown, and covered with short black hairs.
The front feet of A. maximus are small, with relatively short, robust digits bearing long, opaque claws with decurved tips, except the pollex, which is short and bears a nail. The dorsal and palmar surfaces are uniformly pigmented dark grayish brown. The palmar pads consist of three interdigitals, and a thenar and hypothenar, which are larger than the interdigitals. The hind feet are long and slender, with relatively short digits; the longest middle digits including claws are less than one-third the length of the hind foot. The claws are opaque, shorter than on the forefeet and with short decurved tips. Both the dorsal and plantar surfaces, including digits and plantar pads, are pigmented dark grayish brown. The plantar pads are small relative to plantar surface and consist of four interdigitals, a large thenar, and smaller, round hypothenar; the metatarsal is small and rounded; the rest are ovate and larger.
The tail of the holotype of A. maximus is 13% shorter than the combined length of head and body; the paratypes have from 20% shorter to slightly longer tail than the combined length of head and body (table 1). There are ca. 20 tail scale rows/cm (TSR) in the holotype, 20–24 TSR in paratypes; each scale bears three short hairs. Tail is uniformly pigmented dark grayish brown, with its dorsal surface covered with short, similarly pigmented hairs and its ventral surface covered with a mix of dark and pale to unpigmented hairs.
Archboldomys maximus View in CoL and A. luzonensis View in CoL have similarly upturned nasal tips that project beyond the anterior margins of the premaxillae and broad, squarish first molars, and upper incisors with acutely angled posterior edges (figs. 9, 10, 13). However, differences in cranial features and measurements easily distinguish A. maximus View in CoL from A. luzonensis View in CoL (tables 2, 11; figs. 9–13; Musser, 1982a: figs. 13; 22–24, 26–30; Rickart et al., 1998: fig. 12), including: (1) a longer, broadly ovate skull with proportionately longer rostrum (shorter in A. luzonensis View in CoL ) and laterally inflated braincase (less inflated); (2) shorter incisive foramina (longer); (3) elongate interpremaxillary fossa that is placed well behind a line connecting the posterior margins of the incisors (rounded interpremaxillary foramen, close to a line connecting the posterior margins of the incisors); (4) narrow zygomatic plate with a more pronounced slanting anterior edge (wider and less slanting); (5) shorter orbito-temporal length (longer); (6) longer and wider pterygoid ridge extending posteriad to become the pterygoid bridge over the foramen ovale (shorter and narrower, terminating posteriad above alisphenoid strut); (7) and narrow, delicate alisphenoid strut hidden under pterygoid ridge (broad, robust, and conspicuously exposed alisphenoid).
The skull of A. maximus View in CoL is smooth, with gracile, tapered rostrum and dorsolaterally swollen cranium (figs. 3, 9, 13). In lateral view, its dorsal profile is nearly straight from the top of the skull to about the anterior base of the premaxilla, from which the profile assumes a short and smoothly shallow anteriad convexity brought about by the upturned nasal tips. The nasal tip projects beyond the anterior edge of the premaxillae. The slightly raised bony capsule of the upper incisor root terminates medially near the suture with the maxilla, opposite the dorsal opening of the small and narrow lachrymal canal. The opening of this canal slants caudad, and its nearly flat outer wall barely projects beyond the lateral outline of the capsular swelling of the upper incisor root. The zygomatic plate slants dorsad, relative to the upper molar tooth row. The zygomatic process of the squamosal is low on the cranium, anchored less than a millimeter above the dorsal edge of the postglenoid foramen.
The tympanic hook of the squamosal is slender and short relative to cranial size. The squamoso-mastoid foramen is small relative to the postglenoid vacuity and is either obscured entirely by a thin membrane, as in the holotype, or partially obscured ventrally by the wide, posterior extension of the periotic part of the petromastoid. The mastoid of adults has no fenestra (e.g., FMNH 193526, 193943, 193944). In younger adults, a narrow slit in various stages of closure is present in the mastoid (as in the left mastoid of the holotype [the right one is completely ossified], FMNH 193531; fig. 13). A small mastoid foramen is situated along the medial occipital suture.
The incisive foramina of A. maximus are long and narrow, round edged posteriad and smoothly tapered anteriad (fig. 13, table 2). A small interpremaxillary foramen is present, lying visibly posterior to a line connecting the posterior edges of the incisors (fig. 9). The alisphenoid canal is small and circular, bridged by the thin alisphenoid strut, both well concealed under the wide pterygoid ridge. The pterygoid ridge itself is also long, extending posteriad and becoming the pterygoid bridge over the foramen ovale. The postglenoid vacuity is spacious and domed, and the widest part appears nearly round due to the smooth concavity of the posterior periotic part of the petromastoid opposite the domed dorsal edge; in the holotype, the left vacuity is sealed by a membranous covering. The auditory bulla is small relative to cranial area, and anteroventrally inflated (viewed right side up), obscuring the anterior half of the petrosal in ventral view.
Dental features are strikingly similar between A. maximus and A. luzonensis , both sharing slightly ( A. luzonensis ) or strongly ( A. maximus ) ophistodont upper incisor procumbency, upper incisors in ventral view that are sharply angled on the posterior edge (fig. 9), and squarish first upper molars. But several dental and mandibular features and measurements easily distinguish A. maximus from A. luzonensis (figs. 2, 3, 9–14, table 2; Musser, 1982a: figs. 13, 32, 33; Rickart et al., 1998: fig. 12), including longer molariform tooth row (shorter in A. luzonensis ), larger molars with complete cuspidation on the first upper molar (fig. 2; smaller, with absent or missing cusp t3 and cusp t9 on M1), longer and more gracile mandible (shorter and more robust), narrow angular process (wider), and shorter and narrower coronoid process (longer and wider).
The maxillary molars of A. maximus (figs. 2, 14) are marked by their large size. The first upper molar appears squarish due to the conspicuous broadening of the lingual cusps t1 and t4 that together contribute to the width of M1, which is about three-fourths of its length. The cuspidation of the three rows of M1 appears complete (fig. 2), though coalesced in the broad laminar outlines of the deeply basined occlusal surface, cusp t3 on the first row is barely visible at the edge of the worn, broad, and downsloping labial lamina, and cusps t7 and t9 have either merged with t8 or are absent. The second molars are smaller than the first, but the anterolingual convexity of cusps t1 and t4 makes them wider than long, and trapezoidal in occlusal outline. Cusps t2 and t3 are missing on the first row. The second and third rows appear to have complete cuspidation, coalesced in the laminar outlines of their occlusal surfaces. The third molar has less than half the surface area of the second molar, and has a cordate occlusal outline brought about by the broad lingual cusp t1 and what appears to be the anterolabially oriented second row, with presumed cusps t4, t5, and t6 coalesced into the narrow laminar outline of its occlusal surface. The slanting orientation of this second row results in posteriad placement of cusp t4 (figs. 2, 14). No evidence of a third row is discernible.
The dentary of A. maximus is slightly longer and more gracile than in A. luzonensis (figs. 10, 13; Musser, 1982a: fig. 13). The labial surface of the mandible is smooth, and the capsular process is barely discernible. The placement of the mandibular and mental foramina is similar to A. luzonensis . The mandibular molar row (fig. 14B) is slender. The lower incisors are robust, sharply pointed, and the anterior surface covered with pale yellow enamel (fig. 13). The incisor is broadly procumbent, forming a smooth, wide curve from the anterior edge of the first mandibular molar alveolus. The coronoid process is short, slender, and backswept, extending slightly more than halfway from its base to the base of the adjacent condyle. The condyloid process is short and robust. The angular process is slender with a narrowly angular and slightly upturned tip, forming a deep sigmoidal notch with the posterior edge of the condyle.
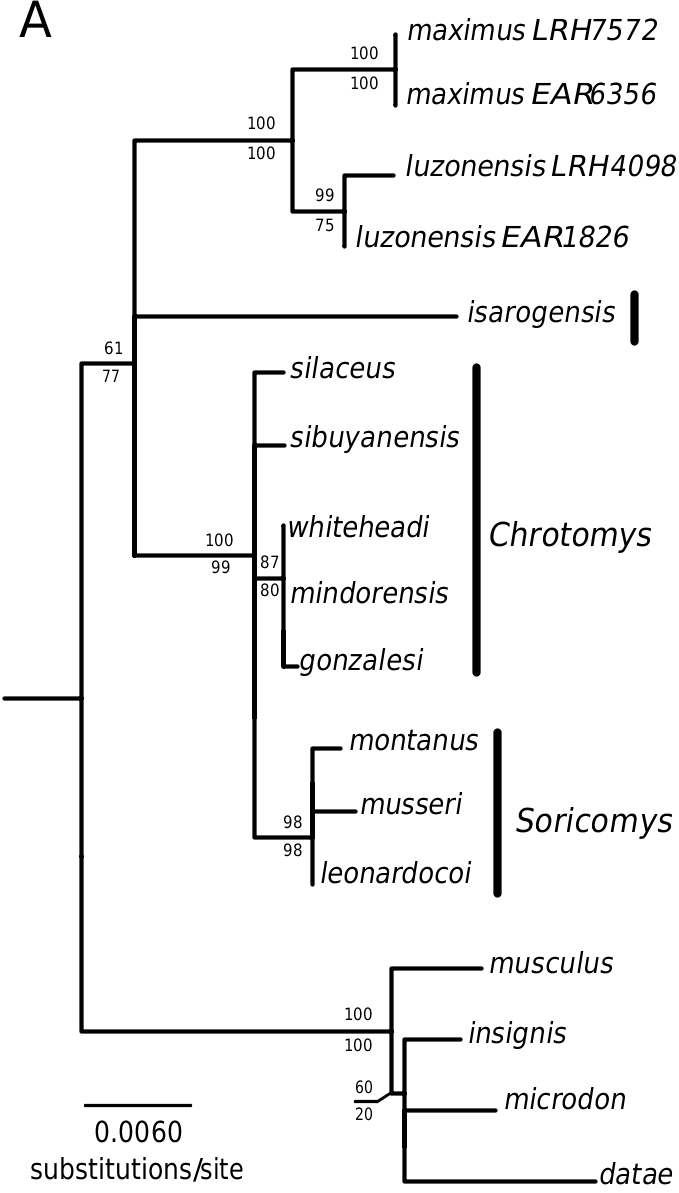
As noted above, a PCA analysis based on cranial and dental measurements (fig. 4, table 5) demonstrated no overlap between A. luzonensis and A. maximus on the second axis, with A. maximus having a proportionally longer skull, more elongate diastema and postpalatal region, and broader upper incisors measured near the tips, and A. luzonensis having the converse. Analysis of the mitochondrial cytochrome b and IRBP nuclear gene gave strong support to these two species as sister taxa, with branch lengths separating them similar to those separating the species of Soricomys (figs. 7, 8).
DISTRIBUTION: Currently known only from Mt. Amuyao, Mountain Province, in the Central Cordillera of northern Luzon (figs. 1, 15; Rickart et al., 2011b). However, Mt. Amuyao is the only place in the southern portion of the Central Cordillera where surveys in the relevant habitat (mature montane and mossy forest) using the relevant sampling methods (traps baited with live earthworms) have been conducted. The species may occur in montane and mossy forest over a broad portion of the Central Cordillera; additional surveys are needed to determine the extent of its distribution.
ECOLOGY: Archboldomys maximus has been captured in old-growth montane and mossy forest, from 1885 m to 2690 m ( Rickart et al., 2011b). There were slightly more diurnal (8) than nocturnal/crepuscular (5) captures. Success with traps baited with live earthworms was higher than with fried coconut, but the effect was not statistically significant. Stomach analysis suggests a predominantly vermivorous feeding habit; stomach contents of six individuals all included earthworms, in addition to centipedes (order Geophilomorpha ) in three individuals, and a few fragments of insect exoskeletons (body and integument of adults and larvae) in four individuals.
Females have two pairs of mammae, both inguinal. Reproductive activity early in the year was apparent. Five of six males caught in March had scrotal testes, one of which had a testes size of 10 × 7 mm with convoluted epididimys; during the same period, two of seven females had large mammae, one of which was pregnant with a single embryo and had one placental scar (Balete, Heaney, and Rickart field notes in FMNH).
Eleven other species of nonvolant small mammals were recorded in montane and mossy forest habitat with Archboldomys maximus : Crocidura grayi , Apomys datae , A. musculus , Batomys granti , Bullimus luzonicus , Chrotomys silaceus , C. whiteheadi , Musseromys sp. , Rattus everetti , Rhynchomys soricoides , and Soricomys montanus , new species ( Rickart et al., 2011b). Among them, six are members of the Chrotomys Division: Apomys datae , A. musculus , S. kalinga , C. silaceus , C. whiteheadi , and R. soricoides (table 12). Mt. Amuyao is the only place known currently where species of Archboldomys and Soricomys , n. gen., occur sympatrically. Additional species of native nonvolant mammals we documented on Mt. Amuyao included Apomys abrae (at lower elevations), and the giant cloud rats Crateromys schadenbergi and Phloeomys pallidus ( Rickart et al., 2011b) .
Soricomys , new genus
TYPE SPECIES: Archboldomys kalinga (Balete, Rickart, and Heaney, 2006) .
ETYMOLOGY: From the Latin sori - (sorex, “shrew”), in combination with mys (Gk., “mouse”), for its close similarity to the shrews in body form and habits. Gender masculine.
INCLUDED SPECIES: As currently understood, S. kalinga , S. leonardocoi , n. sp., S. montanus , n. sp., and S. musseri . See comments below.
DISTRIBUTION: Luzon Island only; currently known only from the Central Cordillera, the Mingan Mountains, and the northern Sierra Madre (fig. 1).
DIAGNOSIS: A genus of small-bodied shrew mice, superficially similar to Archboldomys in external morphology, but distinguished from it and other known murid rodents by the following combination of external, cranial, and dental features (table 11): (1) dorsal fur darker and longer than ventral fur; (2) short, tapered rostrum; (3) straight nasal tips terminating above the anterior margins of the premaxillae; (4) straight anterior edge of zygomatic plate relative to the horizontal molar row; (5) short and broad tympanic hook; (6) narrow to nearly closed squamoso-mastoid foramen; (7) prominent mastoid fenestra; (8) orthodont upper incisors procumbent; (9) broadly angled to nearly rounded lingual edge of upper incisors; (10) narrow molar row; (11) relatively narrow, rectangular upper first molars, M1; and (12) interpremaxillary foramina (and presumably the vessel it transmits) absent (only minute nutrient foramina present; fig. 9).
COMMENTS: Comparisons to Archboldomys are given above.
Soricomys kalinga (Balete et al., 2006) View in CoL and S. musseri ( Rickart et al., 1998) View in CoL were both originally described as members of Archboldomys View in CoL . Several cranial and dental characters highlighted during the original diagnosis of S. musseri View in CoL , including straight nasal tips, short incisive foramina, small postglenoid vacuities, prominent mastoid fenestra, and smaller molars, are now among the diagnostic features of Soricomys View in CoL (table 11). Also, as noted in the original description, the ventral pelage of S. musseri View in CoL is slightly shorter and paler than the dorsal fur (with a gradual
Location of mountains indicated in figure 1. Except for A. abrae View in CoL and C. mindorensis View in CoL , species present on each mountain were recorded in the same habitat and elevation as with Archboldomys View in CoL and Soricomys View in CoL . transition between them), in contrast to the uniformly colored pelage of A. luzonensis View in CoL . The discovery of the three new species, A. maximus View in CoL , S. leonardocoi View in CoL , n. sp., and S. montanus View in CoL , n. sp., demonstrates the consistency of these differences.
Phylogenetic analysis of mitochondrial cytochrome b and nuclear IRBP genes gave strong support to these four species as a clade, with an unresolved trichotomy among S. musseri , S. leonardocoi , n. sp., and a branch containing S. kalinga and S. montanus , n. sp. Although we do not advocate using threshold genetic divergence values as a way to diagnose species, we note that divergence values among species of Soricomys are comparable to or higher than those among other currently recognized species of this group. Notably, average uncorrected (p) sequence divergence for cytochrome b ranges from 7.9% to 8.9% among these four species of Soricomys , which is similar to the divergence between the two species of Archboldomys (9.1%); within the range of divergence values among species of Chrotomys (2.8% to 11.7%); and much higher than the divergence values observed among recognized species of Rhynchomys (1.4% to 3.1%; fig. 7).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Archboldomys luzonensis Musser, 1982
| Balete, Danilo S., Rickart, Eric A., Heaney, Lawrence R., Alviola, Phillip A., Duya, Melizar V., Duya, Mariano Roy M., Sosa, Timothy & Jansa, Sharon A. 2012 |
Archboldomys maximus
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
A. maximus
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
A. maximus
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
Soricomys
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
Soricomys
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
Soricomys
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
A. maximus
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
S. leonardocoi
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
S. montanus
| Balete & Rickart & Heaney & Alviola & Duya & Duya & Sosa & Jansa 2012 |
A. luzonensis
| Musser 1982 |
A. luzonensis
| Musser 1982 |
A. luzonensis
| Musser 1982 |
Archboldomys
| Musser 1982 |
Archboldomys
| Musser 1982 |
Archboldomys
| Musser 1982 |
A. luzonensis
| Musser 1982 |