Spiniglans thomassankara, Mariaux, 2021
|
publication ID |
https://doi.org/ 10.35929/RSZ.0057 |
|
persistent identifier |
https://treatment.plazi.org/id/502587BB-096F-4D23-F6DA-CAD6BD31D654 |
|
treatment provided by |
Felipe |
|
scientific name |
Spiniglans thomassankara |
| status |
sp. nov. |
Spiniglans thomassankara sp. nov.
Figs 1-5 View Figs 1-5
Holotype: MHNG-PLAT-0015962 (formerly MHNG 988.402); Côte d’Ivoire, District Autonome d’Abidjan, Songon , fields ab. 7 km E of Dabou; WGS84 5.33, -4.31, 3 m; 21.04.1988. From Ploceus nigerrimus castaneofuscus (Lesson, 1840) ( Passeriformes , Ploceidae ); MHNG-OIS-1774.026 .
Paratype: MHNG-PLAT-0015941 (formerly MHNG 985.637 View Materials ); 1 specimen; Côte d’Ivoire, District Autonome d’Abidjan , Adiopodioumé, former Orstom Experimental Fields; WGS84 5.33, -4.14, 45 m; 24.10.1985. From Ploceus nigerrimus castaneofuscus ( Passeriformes , Ploceidae ); MHNG-OIS-1774.014.
Other material:
- From Ploceus nigerrimus castaneofuscus MHNG-PLAT-0137397-8, MHNG-PLAT-0137400-1; 4 specimens; Côte d’Ivoire, Comoé, Nouamou, along Western end of Ehy Laguna; WGS84 5.18, -2.90, 17 m; 03.06.2010 - 06.06.2010. MHNG-OIS-1982.041-2, MHNG-OIS-1982.046.
- From Ploceus cucullatus (Statius Muller, 1776) MHNG-PLAT-0137402 ; 3 specimens. Same locality as paratypes. 09.06.2010 .
- From Ploceus nigricollis brachypterus Swainson, 1837 .
MHNG-PLAT-0015956 (formerly MHNG 987.290); 2 specimens. Same locality as paratypes. 27.04.1987. MHNG-OIS-1774.050 .
- From Ploceus sp.
MHNG-PLAT-0015951 (formerly MHNG 985.636); 1 specimen. Same locality as paratypes; 23.07.1985 .
Comparison material studied: MHNG-PLAT- 0040450, 5 slides. Spiniglans microsoma ( Southwell, 1922) , “cotypes”.
Prevalences: 7/36 (19%) in P. nigerrimus ; 1/16 (6%) in P. nigricollis ; 1/42 (2%) in P. cucullatus .
Intensity: 1-3.
Etymology: The specific epithet (a noun in apposition) honours, and is dedicated to, Thomas Isidore Noël Sankara (1949-1987), former president of the Burkina Faso, who was enlightening the West Africa subregion when these specimens were collected.
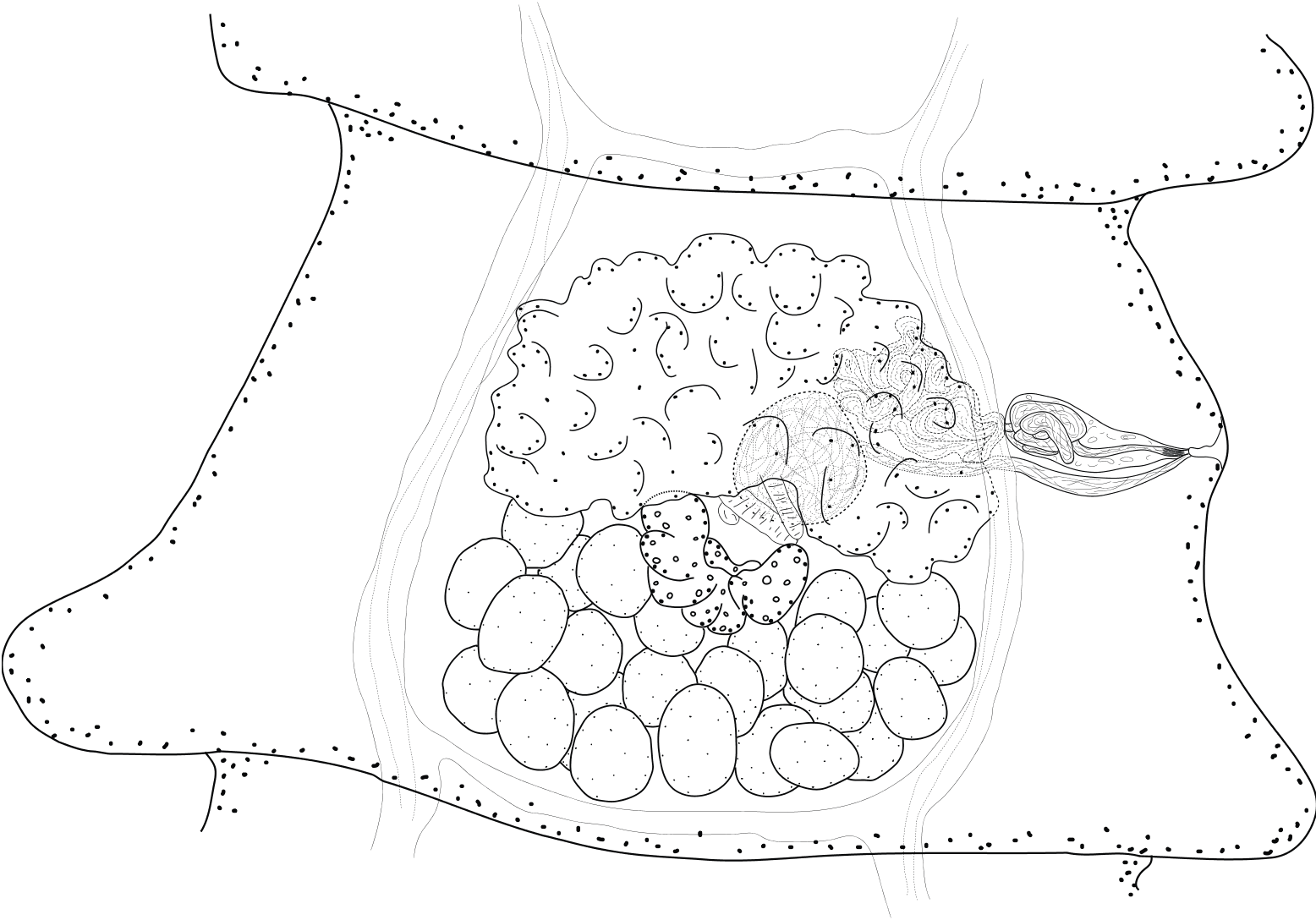
Description: Worm with body of medium size, up to 78 mm in length and with maximum width of 800 at level of early gravid proglottides. Longest specimen with 222 proglottides. Proglottides craspedote, wider than long, except for a few final gravid ones that can be up to twice longer than wide. Scolex rounded, not markedly delineated from neck, 295-490 in diameter (367, n = 10) [312]. Suckers rounded, muscular, unarmed, 118-160 (142, n = 40) [135-145] in diameter. Rostellar sac regular, cylindrical, weakly muscular, extending well past level of posterior margin of suckers into anterior strobila, 342-457 x 123-175 (390 x 146, n = 10) [342 x 123]. Rostellum elongated, very muscular, with well developed glandular tissue, especially in it posterior half, 258-358 x 86-133 (323 x 112, n = 10) [290 x 186] ( Fig. 1 View Figs 1-5 ). Hooks on two rows, 24-26 [26] in number; similar in shape in both rows with long handles and blades, and well developed guards, that are forward oriented. Hooks of first row slightly shorter and straighter than those of second row. Hooks length 62-65.5 on first row (64, n = 13) and 66.5-69.5 on second row (68, n = 11) ( Fig. 2 View Figs 1-5 ). Neck poorly marked; proglottization distinct at 400- 550 from posterior margin of suckers. Genital pores lateral, situated slightly anterior to mid-length of lateral proglottis margin, irregularly alternating in very short series, e.g. …, 3, 2, 3, 2, 2, 1, 2, 1, 2, 1, 1, 1, 2, 1…; or …, 2, 1, 1, 1, 1, 4, 2, 1, 1, 2, 1, 1, 1, 3, …, no more than 5 consecutive pores observed on a single side. Ventral osmoregulatory canals up to 55 wide, possibly more in terminal proglottides, connected posteriorly in each proglottis by transverse anastomosis. Dorsal osmoregulatory canals 5-12 wide. Genital ducts passing between osmoregulatory canals. Genital atrium small, unremarkable, sink-shaped, up to 20 deep and 30 in diameter.
Testes numerous, 24-34 (29.5, n = 40) [25-30] in number; in 2-3 layers, in one continuous, transversely elongated posterior field, may occasionally overlap posterior lobes of ovary or osmoregulatory canals ( Fig. 3 View Figs 1-5 ). External vas deferens convoluted antero-porally, behind proximal extremity of cirrus-sac. Cirrus-sac weakly muscular, straight and elongate, 96-140 x 30-45 (114 x 37, n = 44) [101-112 x 36-40]; most commonly not reaching osmoregulatory canals, or just overlapping them. Internal vas deferens forming several coils. Cirrus armed with typical terminal dense tuft of hair, reaching 20-31 (26, n = 28) in length. No evaginated cirrus observed. Ductus masculinus very short, often difficult to see or absent ( Fig. 4 View Figs 1-5 ).
Vitellarium central, compact with irregular large lobes, transversely elongated, variable in shape, most often forming a flattened V, and becoming straighter in older mature proglottides; reaching up to 325 x 65. Ovary, antero-central, transversely elongated, mostly compact with poorly marked lobules, reaching longitudinal osmoregulatory canals on both sides. Mehlis’ gland subglobular, anterior to vitellarium. Seminal receptacle round, becoming more oval when full but never elongated; reaching up to 185 x 115 in late mature and pregravid proglottides; dorsal and between ovary wings, anterior to poral part of vitelline gland. Vagina opened posteriorly to male pore, very long, straight and transverse, parallel and ventral to cirrus-sac; thick-walled; not divided into copulatory and conductive part; surrounded by a loose irregular sheath; no vaginal sphincter ( Fig. 3 View Figs 1-5 ).
Uterus starts its development in late mature proglottides as a diffuse ventral reticulum, progressively forming numerous small lobes and occupying entire median field; crossing osmoregulatory canals and extending in lateral fields. Uterus eventually becoming sacciform with deep, progressively disappearing septa. Eggs oval with thick embryophores 41-54 x 35-48 (49 x 40, n = 22). Oncospheres 26-36 x 20-28 (30 x 24, n = 25). No polar processes. Central embryonic hooks slightly longer 16- 18 (17, n = 14), and thinner than lateral ones 14-16 (15, n = 20). Lateral embryonic hooks similar within each pair ( Fig. 5 View Figs 1-5 ).
Remarks: This material has first been reported, albeit without discussion, in Mariaux (1994) under the name
Anomotaenia sp. Meanwhile Bona (1994) proposed a comprehensive synthesis of the Dilepididae systematics, which, essentially, is still accepted today ( Mariaux et al., 2017).
In Bona (1994), the group of genera defined by the presence of tufts of bristle-like spines on the cirrus is well defined but its organization is particularly challenging. Generic diagnoses are complex and sometimes based on difficult to observe or non-classical characters. The present material shows affinities both with Dictymetra Clark, 1952 and Spiniglans Yamaguti, 1959 . It resembles Dictymetra in having clearly defined rows of rostellar hooks with comparatively long blades; no, or very poorly marked, ovary isthmus; and rather long cirrus bristle. On the other hand the following characters make it closer to the definition of Spiniglans : proglottides generally wider than long; very long vagina; extremely short, often not visible ductus masculinus; testes in transverse field and, possibly, absence of polar process in the eggs (although the latter character is certainly variable in Dictymetra , especially for its terrestrial species).
Clearly, the generic placement of this material may consequently only be provisional until this whole group of species is reassessed. In the meantime, I favor a placement in Spiniglans , essentially because of the nearly complete absence of ductus masculinus. A comparison with members of both genera remains nevertheless obviously needed.
Dictymetra and Spiniglans have recently been reviewed and discussed by Mariaux & Georgiev (2018) who also provided a list of accepted species for both genera. They noted the heterogeneous host spectrum of Dictymetra and its possible paraphyly. Indeed, Dictymetra is mostly found in Charadriiformes worldwide, although a few species have also been recorded in terrestrial birds, including one in Passeriformes . The 13 known species in the genus show a global distribution, including in Africa. The present material differs from all species listed by Mariaux & Georgiev (2018, see their table 4) mostly by its rostellum size, length of hooks and number of testes. It is however similar to D. belopolskajae Spasskaya & Spasskii, 1973 , a parasite of Alaudidae in Russian Arctic ( Spasskaya & Spasskii, 1973), but can be distinguished from it by showing 24-26 rostellar hooks (vs. 20 in D. belopolskajae ).
Members of Spiniglans are almost all found in Corvidae , from Europe to Australia, but the type species, Spiniglans microsoma ( Southwell, 1922) , was described from a ploceid (and an emberezid) in India ( Southwell, 1922). All seven species recognized by Mariaux & Georgiev (2018, see their table 2) differ from the present material by their hooks number and length as well as by their testes number (our observations of S. microsoma type material revealed a slightly smaller scolex and suckers diameters than originally reported but no major discrepancies with the original description). In consequence, it belongs to a new species, Spiniglans thomassankara sp. nov. The genus’ range is extended to Africa and consequently to the complete Old World.
Weavers are common sights throughout Africa and Ploceidae form a well-diversified family with over 100 recognized species. Their cestode fauna is nevertheless practically unknown, with Anomotania quelea ( Mettrick, 1961) from Quelea quelea (Linnaeus, 1758) in Zambia being the only exception I am aware of. Although being quite similar to our material, the latter species differs from it by a smaller number of testes and a longer cirrussac that is lacking any armature ( Mettrick, 1961).
S. thomassankara is locally relatively abundant in its type host (19% prevalence) but much less so in the other two congeneric hosts in which it was found. It has not been found neither in other sympatric Ploceus potential hosts [e. g. P. aurantius (Vieillot, 1808) , 17 examined; P. heuglini Reichenow, 1886 , 2 examined] nor in other common Ploceidae taxa in the same area (e. g. belonging to Malimbus or Vidua ) or other parts of the country (e. g. belonging to Euplectes or Plocepasser ). Lagunae from Southern Ivory Coast constitute a particular, and endangered, ecosystem belonging to the “Guinean mangroves ecoregion” that extend from Senegal to Ivory Coast ( Burgess et al., 2004; Carr et al., 2015). Should S. thomassankara intermediate hosts be linked to this peculiar environment, the species may possibly be endemic to the region.
| MHNG |
Switzerland, Geneva, Museum d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |