Halecium mediterraneum Weismann, 1883
|
publication ID |
https://doi.org/10.1080/00222930400001319 |
|
persistent identifier |
https://treatment.plazi.org/id/4B6087F1-8B5E-FFDC-CB3D-FF6DFE06FCF8 |
|
treatment provided by |
Carolina (2021-04-07 11:05:19, last updated by Plazi 2023-11-02 05:14:02) |
|
scientific name |
Halecium mediterraneum Weismann, 1883 |
| status |
|
Halecium mediterraneum Weismann, 1883 View in CoL
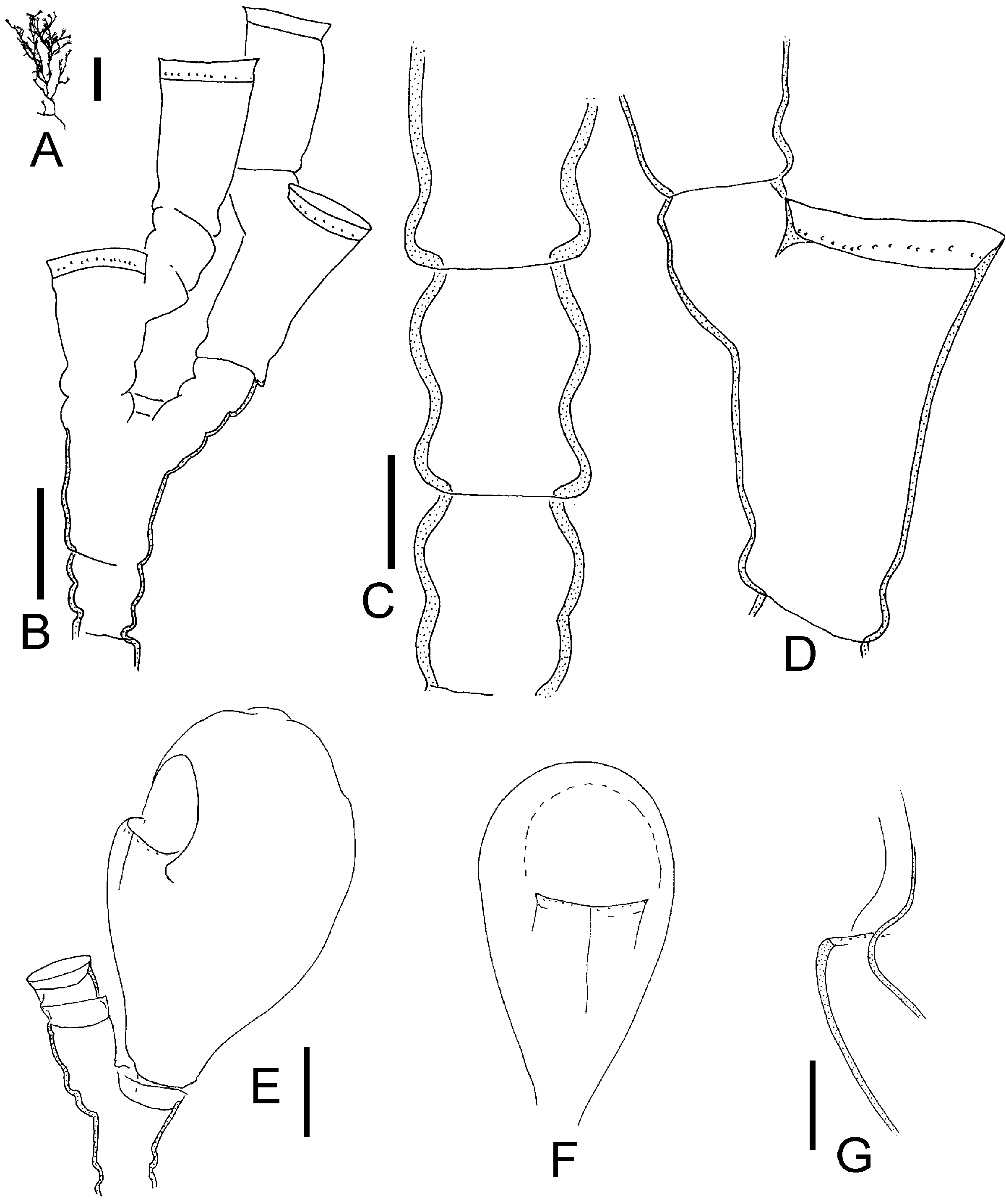
( Figure 11 View Figure 11 )
Halecium tenellum υar. mediteranea Weismann 1883, p 160, Plate 2 Figures 5 View Figure 5 , 6 View Figure 6 .
Halecium gracile: Motz-Kossowska 1911, p 335 View in CoL , Figures 7 View Figure 7 , 8.1 View Figure 8 , Plate 18 Figure 2 View Figure 2 ; Neppi 1921, p 12, Figure 10 View Figure 10 , Plate 1 Figure 10 View Figure 10 .
Halecium flexile: Müller 1914, p 288 View in CoL , Figures 1–3 View Figure 1 View Figure 2 View Figure 3 , Plate 10 Figures 1–7 View Figure 1 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 View Figure 7 .
Halecium mediterraneum: Stechow 1919, p 34 View in CoL ; Gili and Garcia Rubies 1985, p 41, Figure 2K View Figure 2 .
Halecium tenellum: García Corrales et al. 1978, p 9 View in CoL , Figures 1 View Figure 1 , 2 View Figure 2 .
[Not Halecium tenellum Hincks 1861 View in CoL .]
Halecium delicatulum: Patriti 1970, p 23 View in CoL , Figure 20; Ramil Blanco and Iglesias Diaz 1988, p 72, Figure 2 View Figure 2 ; Ramil and Vervoort 1992, p 82, Figure 20a–c. A; Medel and Vervoort 2000, p 12, bibliography; Peña Cantero and García Carrascosa 2002, p 63, Figure 12a, b View Figure 12 .
Material examined
MHNG INVE 26664 , Anse de Troc , Banyuls-sur-Mer, France, Mediterranean, coll. P. Schuchert, 12 July 1999 , fertile female colony. MHNG INVE 26666 , under raft, beach of Banyuls-sur-Mer , coll. P. Schuchert, 4 September 1996 , fertile male colony. MHNG INVE 31115 , Anse de Troc , Banyuls-sur-Mer, coll. P. Schuchert, 23 June 1997 , infertile. MHNG INVE 32955 , between Laboratoire Arago and Anse de Troc, Banyuls-sur-Mer, coll. P. Schuchert, 11 May 2002 , male and female colonies, mass occurrence, examined alive. MHNG INVE 34233 , Santa Lucia, Naples , Italy, 1 m, coll. 14 April 1911 , fertile male. MHNG INVE 34232 , Nisida , Naples, Italy, 1 m, 28 February 1902 , fertile male. MHNG INVE 34230 , Nisida , Naples, Italy, 1 m, 14 February 1911 , fertile female. MHNG INVE 34229 , Nisida , Naples, Italy, 1 m, 7 April 1911 , fertile female. MHNG INVE 34437 , Calanque du Port d’Alon, Bandol , France, Mediterranean, 24 April 2003, 1 m.
Description
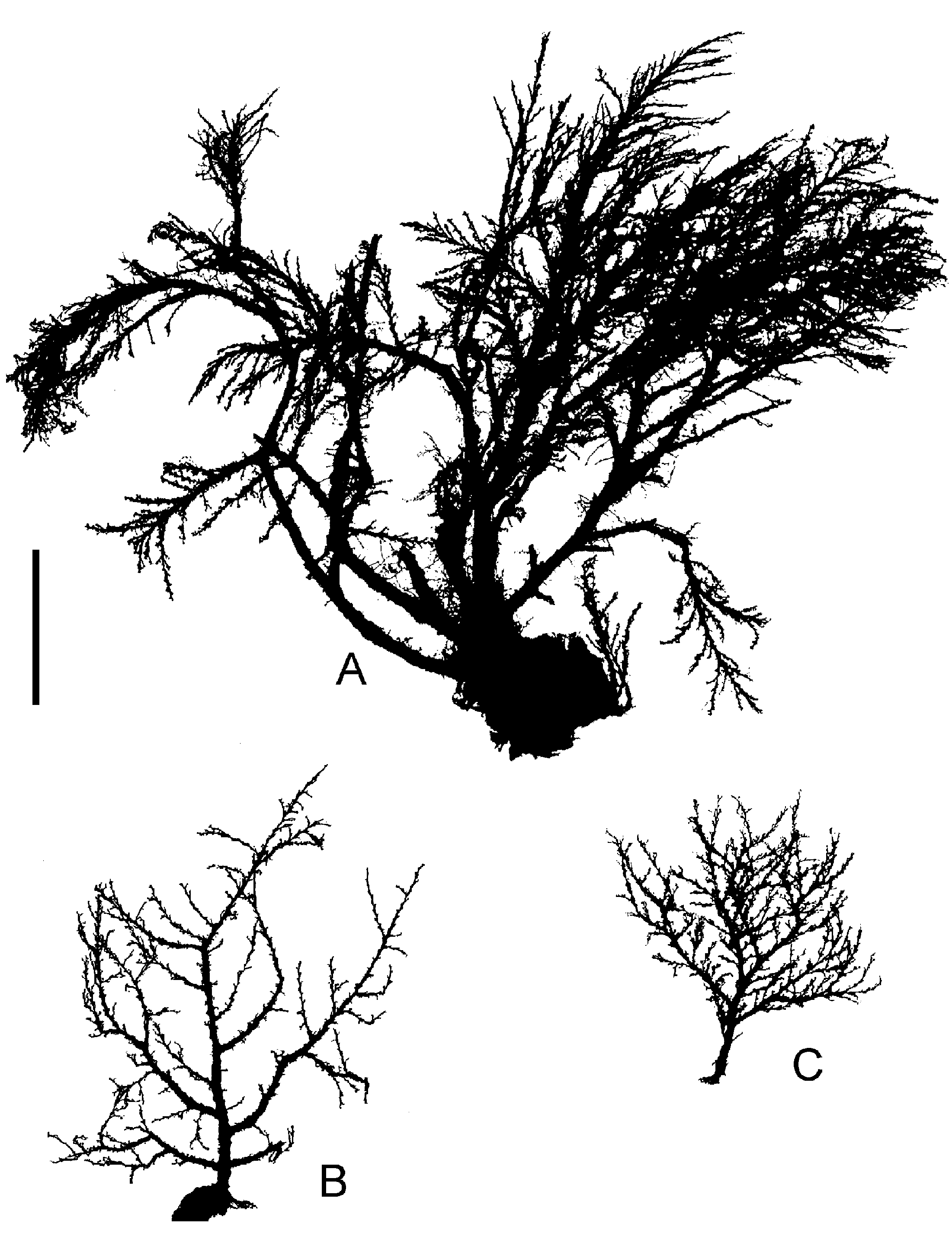
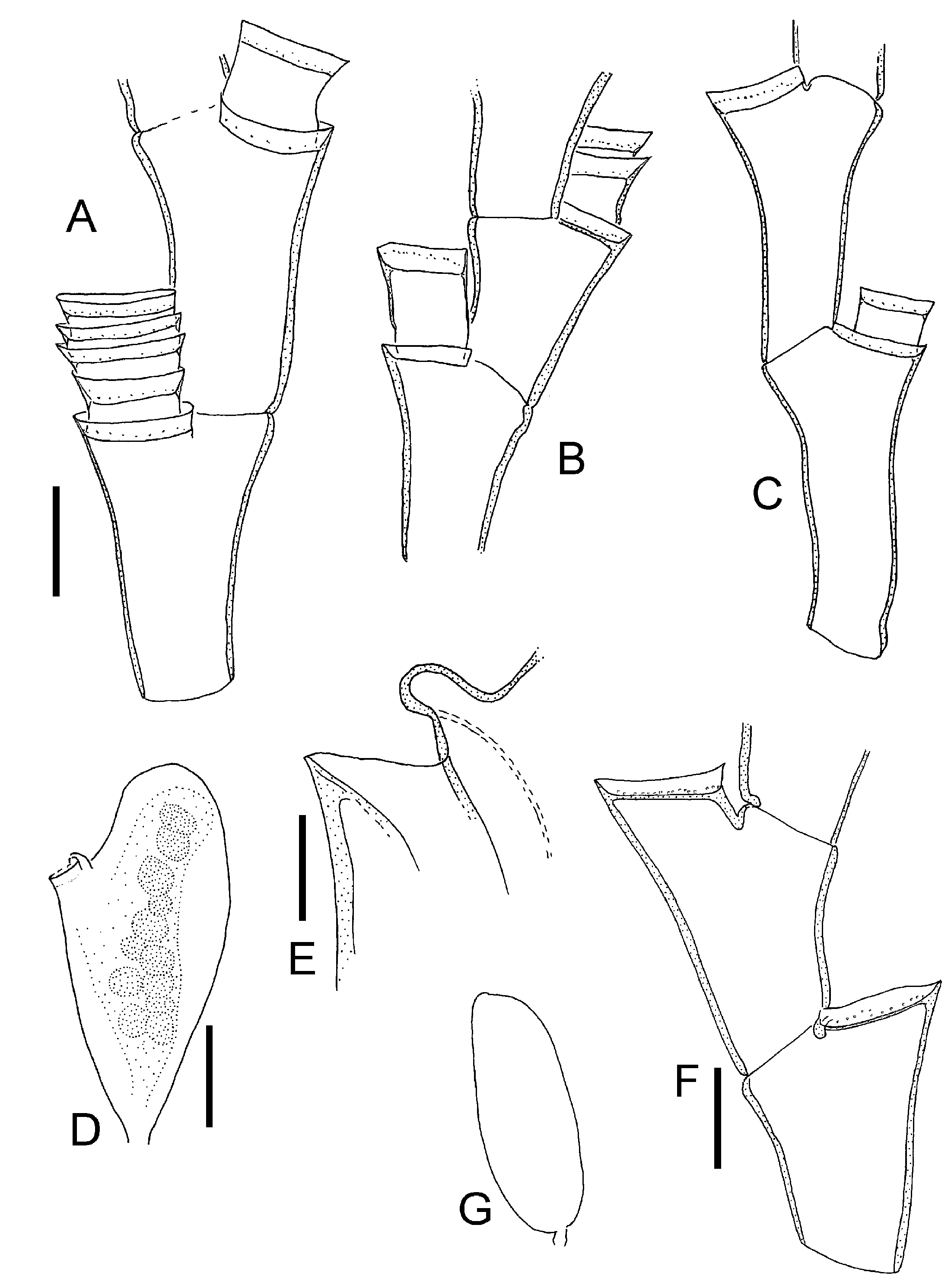
Colonies growing on rock, algae and hydroids, occasionally mass occurrence covering areas of square metres forming a lawn or tangled mass of stems. Stolons tubular, creeping, ramified. Individual shoots 1–3 cm in height, irregularly branched, usually monosiphonic, occasionally weakly polysiphonic, bushy, limp when out of the water, distal parts often geniculate, with regular succession of alternately inclined nodes, nodes distinct. Each internode with a distal hydrophore, oblique, alternately pointing left or right, long and distinctly surpassing level of distal node, hydrophore not delimited by a node. Length of internode 0.4–0.6 mm (mode 0.5 mm), diameter 0.10–0.14 mm, internode length quite homogeneous. Perisarc of internodes smooth, near nodes somewhat bulging, relatively thin. Primary hydrotheca gradually widening and rim somewhat everted or rolled, diameter at base 70–125 mm (mode 80 mm), depth 20–40 mm (mode 30 mm), ring of desmocytes present. Sometimes below diaphragm a semicircular thickening on adcauline side (pseudodiaphragm). Secondary or higher hydrothecae often present, length of their hydrophores as in primary one or longer, often corrugated. Ramification of stems originate from primary hydrophores or from below them; branching usually dichotomous, occasionally trichotomous. Hydranth with 18–20 tentacles. Gonothecae arise in upper axils of branching points and hydrophores; gonothecae dimorphic, the two sexes on separate shoots. Gonothecae of both sexes without protruding hydranths. Female gonothecae smooth, ovoid to rectangular, length 0.7–0.8 mm, breadth 0.5 mm, always compressed but degree variable, along sides a crease-line, distal end obtuse, with or without curved notchlike opening of variable breadth and depth ( Figure 11L, M View Figure 11 ), without distinct lateral ears. Mature female gonothecae have a thin perisarc capsule on inside ( Figure 11L View Figure 11 ). This secondary capsule envelops the gonangium, towards the opening its wall gets thicker. Gonangium oblong, surface epidermis with numerous nematocysts, without hydranth, 6– 13 eggs embedded in tissue of ovoid shape, egg diameter about 0.1 mm. Development to planula takes place within gonotheca (larviparity). Male gonothecae smaller, ovoid, length up to 0.7 mm, compressed, sides with crease, distal end rounded, without notch or inner secondary capsule, opening slit-like. One oblong sperm mass. Living colonies have a characteristic yellow-brown colour; the pigment is extracted in formalin fixative.
Biology
Mature colonies were observed from April to November ( Motz-Kossowska 1911; Peña Cantero and García Carrascosa 2002; own observations). Depth range 0.5–145 m ( Peña Cantero and García Carrascosa 2002).
Distribution
Mediterranean, perhaps also adjacent Atlantic Ocean from Morocco to Galicia. Type locality: Naples, Italy, Mediterranean.
Remarks
Upon closer examination of mature female gonothecae, I noted that there is a delicate tubelike inner capsule enveloping the gonangium. This secondary capsule attaches to the outer capsule along its distal opening ( Figure 11L View Figure 11 ). Such a secondary capsule was also found in H. delicatulum and in H. labrosum , although in the latter it is very thin and difficult to see. Ralph (1958) also found this inner capsule in H. delicatulum .
Halecium mediterraneum View in CoL is almost indistinguishable from Halecium delicatulum View in CoL . Halecium delicatulum View in CoL was first described by Coughtrey (1876) based on colonies found in Dunedin Harbour, New Zealand. Ralph (1958) re-described and revised it and she synonymized several similar nominal species. She also suspected that Halecium mediterraneum View in CoL might be conspecific with it. Rees and Vervoort (1987) agreed and formally synonymized both names. Subsequent studies dealing with Mediterranean collections (e.g. Ramil and Vervoort 1992; Peña Cantero and García Carrascosa 2002) continued this usage. Vervoort and Watson (2003), however, did not synonymize it.
Halecium mediterraneum View in CoL was initially described by Weismann (1883) as a variant of H. tenellum Hincks, 1861 View in CoL . Because it is clearly distinct from H. tenellum, Stechow (1919) View in CoL raised it to full species level.
Comparison of H. mediterraneum View in CoL from the Mediterranean and H. delicatulum View in CoL from New Zealand showed that at least the microscopic structure of their trophosomes is very similar. There were differences in the shape of the female gonotheca (compare Figures 11 View Figure 11 and 12 View Figure 12 ). Ralph (1958) documented the variability of female gonothecae and her figures also show shapes as found here in H. mediterraneum View in CoL . Therefore, the differences apparent in Figures 11 View Figure 11 and 12 View Figure 12 are perhaps not really representative. There remain only some slight differences in egg numbers per gonotheca, colony colour and colony form. Because of the slight differences, and mainly for biogeographic reasons, both nominal species are here kept separate, although it is acknowledged that they are very similar. Genetic methods might hopefully clarify whether they really belong to the same biological species.
Among the European Halecium species, H. mediterraneum is uncomfortably intermediate between Halecium labrosum and H. tenellum and it is not always easy to draw a dividing line. This may have led García Corrales et al. (1978) to synonymize H. tenellum and H. delicatulum . This proposal has been rejected already by other authors (e.g. Ramil and Vervort 1992) and also the present author agrees that they are distinct. Halecium tenellum is more gracile and forms smaller colonies, it is sparingly branched only, the internodes appear more elongate, and it never forms polysiphonic colonies. Fertile Halecium labrosum , in contradistinction, are always polysiphonic, their gonothecae are about twice as large and their shape differs slightly (compare Figures 10E, F View Figure 10 and 11 View Figure 11 E–G). Halecium labrosum often also has a characteristically undulated perisarc ( Cornelius 1995), but this is not a diagnostic feature as the perisarc can be entirely smooth. These differences are valid for European populations only. Halecium mediterraneum may occasionally have been misidentified as H. labrosum . Colonies growing epizoically on dead stems of Eudendrium sp. can feign a strong polysiphonic stem and are prone to be confounded.
Another species within this species cluster is Halecium textum Kramp, 1911 (see below). The main trait to distinguish H. textum from H. mediterraneum is its undulated or corrugated perisarc. There are also differences in its branching pattern (frequent trifid branching) and the more pointed gonothecae. The distributions are disjunct. Halecium textum is an arctic or northern boreal species. Although I am convinced that they are good species, the diagnosis of the limits of all four species remains difficult due to the absence of clearly apomorphic characters.
Cornelius PFS. 1995. North-west European thecate hydroids and their medusae. Part 1. Introduction, Laodiceidae to Haleciidae. Synopses of the British Fauna New Series 50: 1 - 347.
Coughtrey M. 1876. Critical notes on the New Zealand Hydroida, suborder Thecaphora. Annals and Magazine of Natural History (4) 17: 22 - 32.
Garcia Corrales P, Aguirre Inchaurbe A, Gonzalez Mora D. 1978. Contribucion al conocimiento de los hidrozoos de las costas espanolas: Parte 1, halecidos, campanularidos y plumularidos. Boletin del Instituto Espanol de Oceanografia 4: 3 - 73.
Gili JM, Garcia Rubies A. 1985. Contribution a la connaissance de la faune d'hydropolipes de l'ile de Majorque. Anales de Biologia 3: 37 - 53.
Hincks T. 1861. A catalogue of the zoophytes of South Devon and South Cornwall. Annals and Magazine of Natural History (3) 8: 152 - 161, 251 - 262, 290 - 297.
Kramp PL. 1911. Report on the hydroids collected by the Danmark Expedition at North-East Greenland. Meddelelser om GrOnland 45: 341 - 396, Plates 20 - 25.
Medel MD, Vervoort W. 2000. Atlantic Haleciidae and Campanulariidae (Hydrozoa, Cnidaria) collected during the CANCAP and Mauritania-II expeditions of the National Museum of Natural History, Leiden, The Netherlands. Zoologische Verhandelingen 330: 1 - 68.
Motz-Kossowska S. 1911. Contribution a la connaissance des hydraires de la Mediterranee occidentale: II, Hydraires calyptoblastiques. Archives de Zoologie Experimentale et Generale 6: 325 - 352.
Muller K. 1914. Die Regeneration der Gonophoren bei den Hydroiden und anschliessende biologische Beobachtungen, Teil 2: Thecata. Wilhelm Roux Archiv fur Entwicklungsmechanik der Organismen 38: 288 - 363.
Neppi V. 1921. Nuove osservationi sui polipi idroidi del Golfo di Napoli. Pubblicazioni della Stazione Zoologica di Napoli 3: 1 - 31.
Patriti G. 1970. Catalogue des cnidaires et ctenaires des cotes Atlantiques marocaines. Travaux de l'Institut scientifique cherifien, Serie zoologie 35: 1 - 149.
Pena Cantero AL, Garcia Carrascosa AM. 2002. The benthic hydroid fauna of the Chafarinas Islands (Alboran Sea, western Mediterranean). Zoologische Verhandelingen 337: 1 - 180.
Ralph PM. 1958. New Zealand thecate hydroids: Part II, Families Lafoeidae, Lineolariidae, Haleciidae and Syntheciidae, Transactions of the Royal Society of New Zealand, 85: 301 - 356.
Ramil Blanco F, Iglesias Diaz A. 1988. La familia Haleciidae (Cnidaria, Hydrozoa) en las costas de Galicia. Thalassas 6: 71 - 78.
Ramil F, Vervoort W. 1992. Report on the Hydroida collected by the ' BALGIM' expedition in and around the Strait of Gibraltar. Zoologische Verhandelingen 277: 1 - 262.
Rees WJ, Vervoort W. 1987. Hydroids from the John Murray Expedition to the Indian Ocean, with revisory notes on Hydrodendron, Abietinella, Cryptolaria and Zygophylax (Cnidaria: Hydrozoa). Zoologische Verhandelingen 237: 1 - 209.
Stechow E. 1919. Zur Kenntnis der Hydroidenfauna des Mittelmeeres, Amerikas und anderer Gebiete, nebst Angaben uber einige Kirchenpauer'sche Typen von Plumulariden. Zoologische Jahrbucher. Abteilung fur Systematik, Geographie und Biologie der Tiere 42: 1 - 172.
Vervoort W, Watson JE. 2003. Leptothecata (Cnidaria: Hydrozoa) (thecate hydroids). NIWA Biodivversity Memoir 119: 1 - 538.
Weismann A. 1883. Die Entstehung der Sexualzelllen bei den Hydromedusen, Zugleich ein Beitrag zur Kenntnis des Baues und der Lebensgeschichte dieser Gruppe. Jena: Gustav Fischer. 295 p.
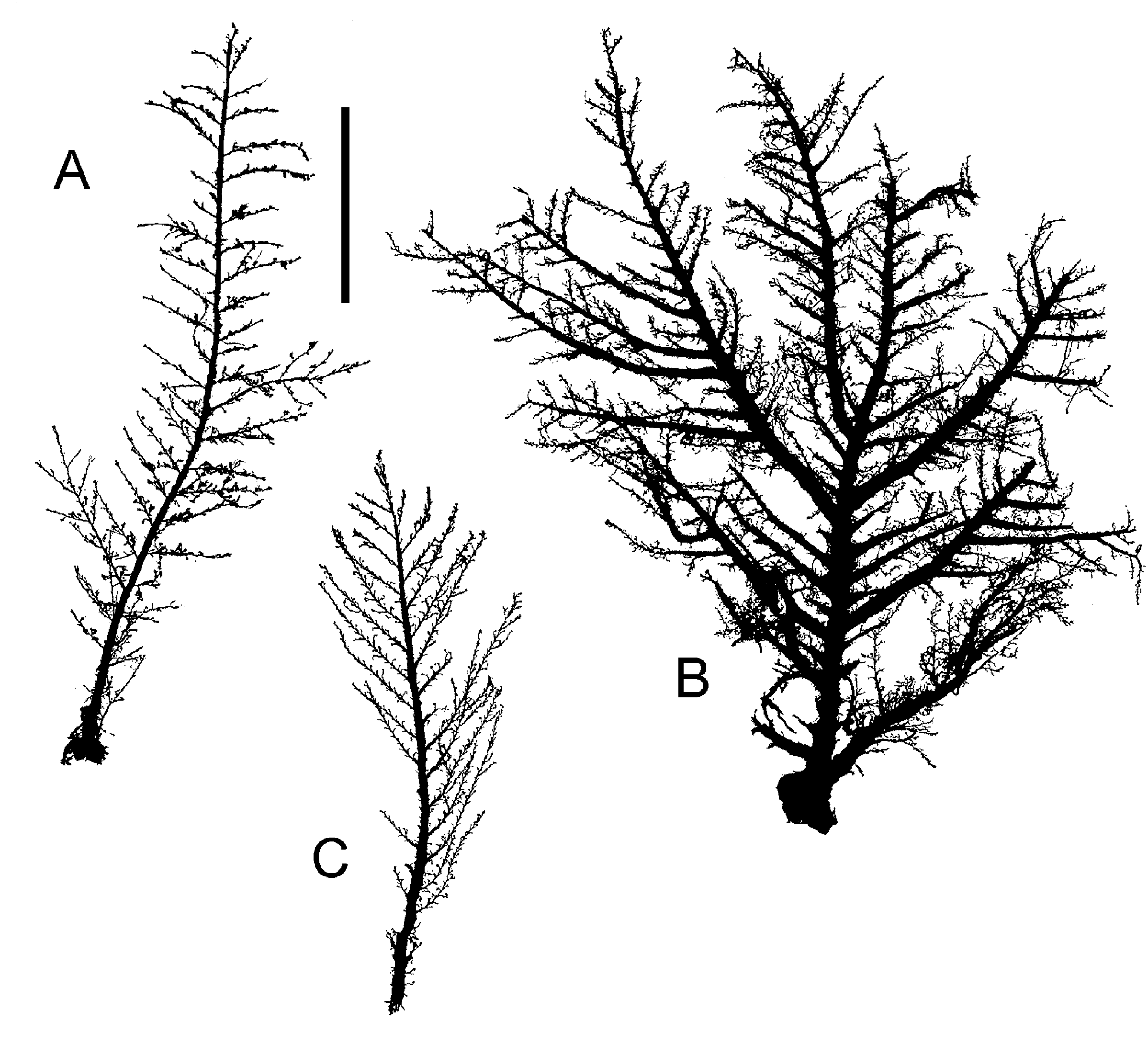
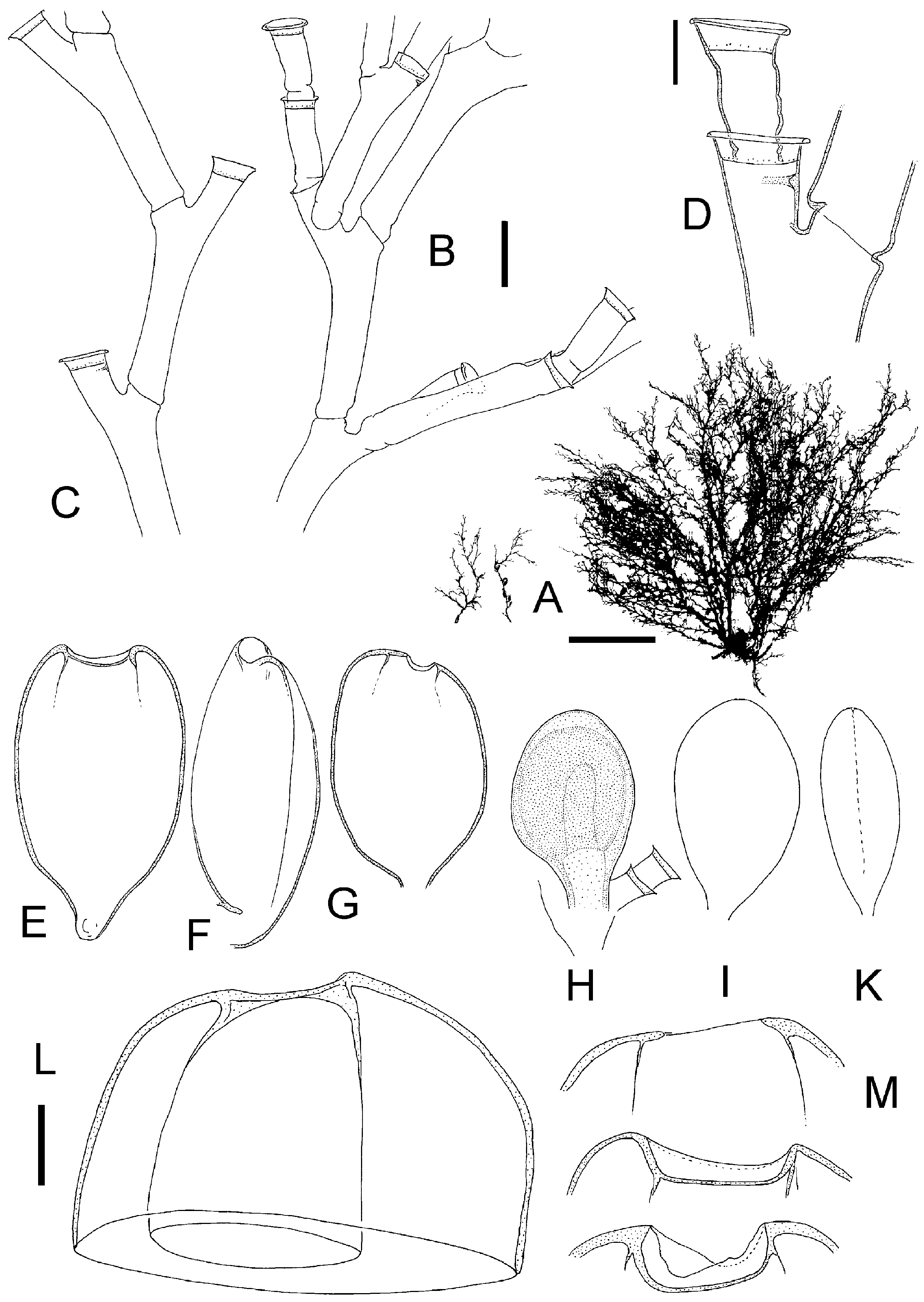
Figure 1. Halecium scutum Clark, 1877; colony silhouettes of material from The Faroes. (A) MHNG INVE 33550; (B) BIOFAR station 699; (C) BIOFAR station 459. Scale bar: 2 cm.
Figure 2. Halecium scutum Clark, 1877. (A) From BIOFAR 699; (B) BIOFAR 556; (C–E) Holsteinsborg, Greenland; (F, G) Augpilagtoq, Greenland. (A–C, F) Segments from monosiphonic parts with primary and sometimes secondary hydrothecae (scale bar: 0.2 mm); (D) female gonotheca in side view, eggs stippled (scale bar: 0.2 mm); (E) higher magnification of gonothecal opening, side view (scale bar: 0.1 mm); (G) male gonotheca (same scale bar as D).
Figure 3. Halecium halecinum (Linnaeus, 1758); colony silhouettes, note variation of shapes. (A) Canary Islands; (B) The Faroes (BIOFAR station 111); (C) The Faroes (BIOFAR station 554). Scale bar: 2 cm.
Figure 4. Halecium halecinum (Linnaeus, 1758). (A–C) Segments of monosiphonic parts, with secondary hydrothecae, note length variation of internodes, BIOFAR station 350, 205 and 351; (D) BIOFAR 351, female gonotheca in side view; (E) as in (D), but seen from anterior side, note bipartite hydrotheca; (F) BIOFAR 597, male gonotheca. Scale bar: 0.2 mm.
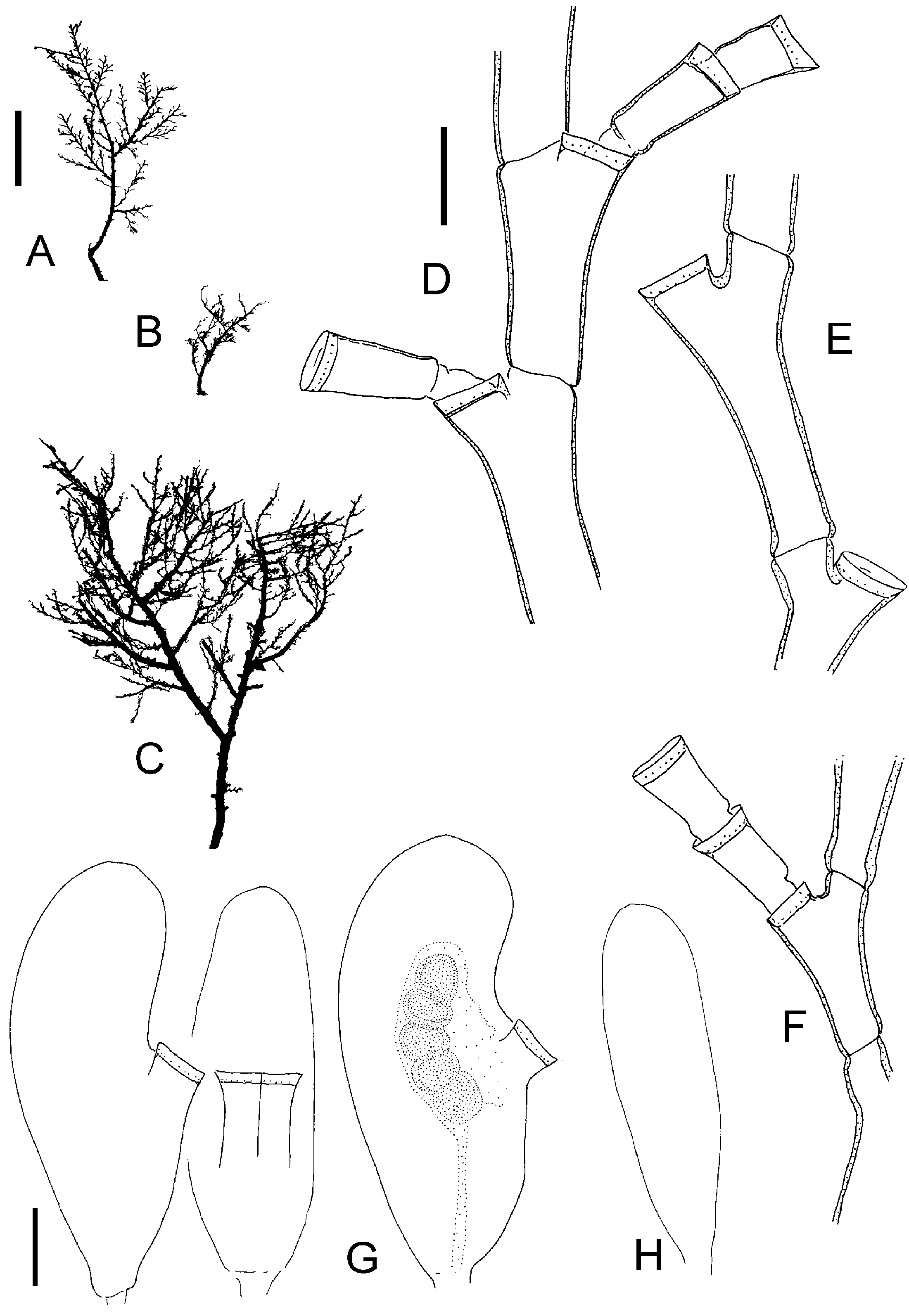
Figure 5. Halecium beanii (Johnston, 1838). (A, D) From Roscoff; (B, C, E–H) from The Faroes. (A–C) Colony silhouettes (scale bar: 1 cm); (D–F) subterminal portions of branches (scale bar: 0.2 mm); (G) female gonotheca, middle one seen from side of hydrothecae, right one shown with developing embryos (scale bar: 0.2 mm); (H) male gonotheca (same scale bar as G).
Figure 6. Halecium beanii (Johnston, 1838). (A–D) After material from South Africa; (E–F) after material from New Zealand. (A) Two internodes (scale bar: 0.2 mm); (B) female gonotheca in oblique view (scale bar: 0.2 mm); (C) cross-section of female gonothecae in about the middle of its height, note polygonal outline (same scale bar as B); (D) three male gonothecae, note variability (same scale bar as B); (E) two internodes (same scale bar as A); (F) female gonotheca (same scale bar as B).
Figure 7. Halecium lankesterii (Bourne, 1890); after material from Brittany. (A) Colony silhouette (scale bar: 5 mm); (B) part of stem (scale bar: 0.2 mm); (C) lower part of stem with characteristic ahydrothecate segments with constriction in middle (scale bar: 0.1 mm); (D) hydrothecate segment (same scale bar as C); (E) female gonotheca in oblique view, shown without hydranths (scale bar: 0.2 mm); (F) female gonotheca seen from anterior side (same scale bar as E); (G) lateral view of female gonotheca, higher magnification of opening (scale bar: 0.1 mm).
Figure 8. Halecium petrosum Stechow, 1919; after material from Banyuls-sur-Mer. (A) Colony silhouette (scale bar: 5 mm); (B) two internodes (scale bar 0.2 mm); (C) branching point (same scale bar as B); (D) hydrophore, primary and secondary hydrothecae (scale bar: 0.1 mm); (E) hydrotheca with straight walls (same scale bar as D); (F) female gonotheca in side view (same scale bar as B); (G) hydrotheca of female gonotheca in anterior view (anterior side in F is directed towards left) (same scale bar as D); (H) part of male colony, sperm masses stippled, note that this sample shows signs of multiple re-growth and regeneration, its identity is not entirely secure (scale bar: 0.2 mm).
Figure 10. Halecium labrosum Alder, 1859. (A–D) MHNG INVE 33563, Iceland; (E–G) MHNG 33583, The Faroes. (A) Colony silhouette (scale bar: 1 cm); (B) segments of distal branch, note short internodes (scale bar: 0.2 mm); (C) gonotheca (scale bar: 0.5 mm); (D) distal opening of gonotheca (scale bar: 0.2 mm); (E) colony silhouette (scale bar: 1 cm); (F) segments of distal branches, note variability of internode length; (G) gonotheca (scale bar: 0.5 mm).
Figure 11. Halecium mediterraneum Weismann, 1883; after preserved Mediterranean material. (A) Silhouettes of three fertile colonies showing variability of growth (scale bar: 1 cm); (B) branching pattern of stem (scale bar: 0.2 mm); (C) distal part with geniculate segments (same scale bar as B); (D) primary and secondary hydrotheca (scale bar: 0.1 mm); (E–G) female gonothecae (same scale bar as B), (E) in frontal view and with broad opening, (F) in side view, (G) most commonly found opening size; (H–K) male gonothecae (same scale bar as B), (H) with soft tissue, (I) empty, (K) in side view; (L) empty female gonotheca cut apart to demonstrate secondary capsule within outer capsule (scale bar: 0.1 mm); (M) range of variation of distal openings of female gonothecae (same scale bar as L).
| MHNG |
Museum d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Halecium mediterraneum Weismann, 1883
| Schuchert, Peter 2005 |
Halecium tenellum: García Corrales et al. 1978 , p 9
| Garcia Corrales 1978: 9 |
Halecium delicatulum
| : Patriti 1970: 23 |
Halecium delicatulum
| : Patriti 1970 |
Halecium delicatulum
| : Patriti 1970 |
H. delicatulum
| : Patriti 1970 |
Halecium mediterraneum:
| Stechow 1919: 34 |
H. tenellum
| , Stechow 1919 |
Halecium flexile: Müller 1914 , p 288
| Muller 1914: 288 |
Halecium gracile:
| Motz-Kossowska 1911: 335 |
Halecium mediterraneum
| Weismann 1883 |
Halecium mediterraneum
| Weismann 1883 |
Halecium mediterraneum
| Weismann 1883 |
H. mediterraneum
| Weismann 1883 |
H. mediterraneum
| Weismann 1883 |
Halecium tenellum
| Hincks 1861 |
H. tenellum
| Hincks 1861 |