Humeromima rufipes (Boheman, 1834)
|
publication ID |
https://doi.org/10.5252/zoosystema2024v46a8 |
|
publication LSID |
lsid:zoobank.org:pub:26836EE8-C9CF-4DC1-A599-6F29AF1D97B5 |
|
DOI |
https://doi.org/10.5281/zenodo.10929012 |
|
persistent identifier |
https://treatment.plazi.org/id/43063560-FFA8-C941-3CCD-25DAFC0FF842 |
|
treatment provided by |
Plazi |
|
scientific name |
Humeromima rufipes (Boheman, 1834) |
| status |
|
Humeromima rufipes (Boheman, 1834) View in CoL
( Figs 1-6 View FIG View FIG View FIG View FIG View FIG View FIG )
Omias rufipes Boheman View in CoL in Schoenherr, 1834: 500. — Rybiński 1901: 141. — Trella 1930: 7 (distribution). — Kuntze & Noskiewicz 1938: 345 (distribution). — Smreczyński 1966: 49 (key, distribution). — Formánek 1904 b: 176 (key, redescription, figure of aedeagus shape). — Kubisz et al. 1998: 267 (distribution).
Omias rufulipes Marseul, 1873: 562 View in CoL (unjustified emendation by virtue of Article 33.2.3).
Omiamima rufipes View in CoL – Silfverberg 1977: 227. — Dieckmann 1980: 197 (key, distribution). — Mazur 2002: 230.
Humeromima rufipes View in CoL – Podlussány 1998: 81. — Borovec 2013: 298. — Yunakov et al. 2018: 273 View Cited Treatment (distribution). — Khrapov & Yunakov 2020: 144 (distribution, figure)
Omias hanaki –[misidentification] Hildt 1893: 231 (distribution).
MATERIAL EXAMINED. — Lectotype (designation: Borovec 2015: 9) ( Fig. 2A View FIG ). Ukraine • ♂: “ Omias / c.[irca] Volhyn.[ia], Bess.[er]” [manuscript by W. Besser’s hand], “Typus [red, printed]”; “LECTOTYPUS Omias rufipes Boh. R. Borovec des. 2013 [red, printed]”; Humeromima rufipes (Boh.) R. Borovec det. 2013”; NHRS-JLKB 000020796 .
OTHER MATERIAL. — Romania • 2 specimens; Botosani County, near Cotusca; 2.VI.2018; J. Krátký leg.; coll. KJC • 5 specimens; Vrancea County, Iresti, 26.XI.1917; [ 45.91°N, 26.95°E]; bottom of eroded ravine, W. Liebmann leg.; SDEI ( Liebmann 1920) ( Fig. 2B View FIG ) GoogleMaps . Ukraine • 1 ♀; Ivano-Frankivsk Province, “6/7 Wl” [= Vovchynets , 6.VII]; SMNHL GoogleMaps • 1 ♂, 2 ♀; Ivano-Frankivsk Province, 5.5 km ESE Burshtyn, Kasova Hora , 15.VI.-28.VII.2020; 49°13’21”N, 24°42’15”E; 295 m a.s.l.; D. Khrapov & R. Panin leg.; steppe; pitfall trap; coll. KhDC, 26278 GoogleMaps • 1 ♀; Ivano-Frankivsk Province, 1.6 km E Kuropatnyky; 22.V-19.VI.2021; 49°17’2”N, 24°40’8”E; steppe; pitfall trap; D. Khrapov leg.; coll. KhDC, 27736 GoogleMaps • 2 ♀; Lviv Province, “IV. Bóbrka” [= Bibrka, Apr.]; SMNHL • 3 ♀; “9/3 Zn” [= Lviv, Znesinnia, 9.III], “1.”; SMNHL • 1 ♀; Lviv Province, “3/5 Zn” [= Lviv, Znesinnia, 3 May]; SMNHL • 2♀; Lviv Province, “3/5 Zn” [= Lviv, Znesinnia, 3 May], “Leopolis; leg. J. Mazurek”; SMNHL • 4 ♀; Lviv Province, “14/5 Zn” [= Lviv, Znesinnia, 14.V] • 1 ♀; Lviv Province, “9/11 Zn” [= Lviv, Znesinnia, 9 Sep.]; SMNHL • 1 ♀; Lviv Province, “18/5 Ph” [= Lviv, Pohulianka, 18 May]; SMNHL • 1 ♀; Lviv Province, Zolochiv, 1.4 km NW Buchyna, Mts. Drancha , 2.VI.-2.VIII.2019; 50°1’51”N, 25°16’58”E; 328 m a.s.l.; steppe; pitfall trap; leg. D. Khrapov & R. Panin; coll. KhDC, 27363 GoogleMaps • 6♀; Lviv Province, 2.2 km NNW Yaktoriv; Mt. Chekhova ; 16.VII.-01.VIII.2019; 49°45’44”N, 24°32’43”E; 260 m a.s.l.; steppe; pitfall trap; leg. D. Khrapov & R. Panin; coll. KhDC, 27364 GoogleMaps • 1 specimen; ibidem, 22.V-27. VI.2020; coll.KhDC, 27365 • 1♂; Lviv Province, Zolochiv, 2 km NW Pidhirtsi, Mt. Mynych ; 20.V-4.VII.2020; 49°57’12”N, 24°57’41”E; 330 m a.s.l.; steppe; pitfall trap; D. Khrapov & R. Panin leg.; coll. KhDC, 27366 GoogleMaps • 1 ♂, 5 ♀; Lviv Province, Zolochiv, 0.5 km E Kyikiv, right bank of Zolochivka Riv.; 6.V-11.VI.2021; 49°46’58”N, 24°58’10”E; steppe; pitfall trap; leg. D. Khrapov & R. Panin; coll. KhDC, 27732 GoogleMaps • 3 ♀; ibidem; MNHN-EC-EC30587, MNHN-EC-EC30588, MNHN-EC-EC30589 • 3 ♀; ibidem; 11. VI.-10. VII.2021; coll. KhDC, 27908 • 2♀; Lviv Province, Zolochiv , 1.5 km SE Trostianets; 7.V-11.VI.2021; 49°47’40”N, 25°3’25”E; steppe; pitfall trap; leg. D. Khrapov & R. Panin; coll. KhDC, 27735 GoogleMaps • 3 ♀; Lviv Province, Zolochiv , 1.2 km W Osovytsia; 7.V-11.VI.2021; 49°47’16”N, 25°3’40”E; 350 m a.s.l.; steppe; pitfall trap; D. Khrapov & R. Panin leg.; coll. KhDC, 27738 GoogleMaps • 1 ♀; Zolochiv , 1 km SSE Luka; 6.V-12.VI.2021; 49°46’24”N, 25°1’14”E; 318 m a.s.l.; steppe; pitfall trap; D. Khrapov & R. Panin leg.; coll. KhDC; 27761 GoogleMaps • 1 ♀; idem, 12.VI-10.VII.2021; coll. KhDC, 26028 GoogleMaps • 1 ♀; Lviv, Mokrotyn, Mt. Harai ; 28.IV-31.V.2021; 50°0’53”N, 23°57’45”E; meadow; pitfall trap; D. Khrapov & A. Zatushevsky leg.; coll. KhDC; 27719 GoogleMaps • 3 ♀; Ternopil Province, “Каменецъ-ПоÃоAьскъ, В. и И. Якубовския 1907” [= Kamianets-Podilskyi; V. Jakubowski & I. Jakubowski leg.; 1907]; ZIN • 1 ♀; Ternopil Province, “ Volhyn. ”, “coll. Kraatz ”, “rufipes / det. Formánek”, “ Omias rufipes Boh. ”, “SDEI Coleoptera # 303533”; SDEI .
Uncertainly labeled specimen. 1 ♂; “ rufipes / Austria / Schröder”, “ rufipes / det. Formánek”, “ Hyperomias rufipes Boh. ”, “SDEI Coleoptera # 303535 ”; SDEI • 1 ♀;
Mislabeled specimen. “ Sibiria ”, “rufipes / det. Formánek”, “coll. Stierlin ”, “SDEI Coleoptera # 303532 ”; SDEI.
REDESCRIPTION
Measurements
BL = 2.13-2.70 mm, BW = 1.08-1.43 mm, BH = 0.88- 1.18 mm. (detailed measurements of each individuals are available in Appendix 3)
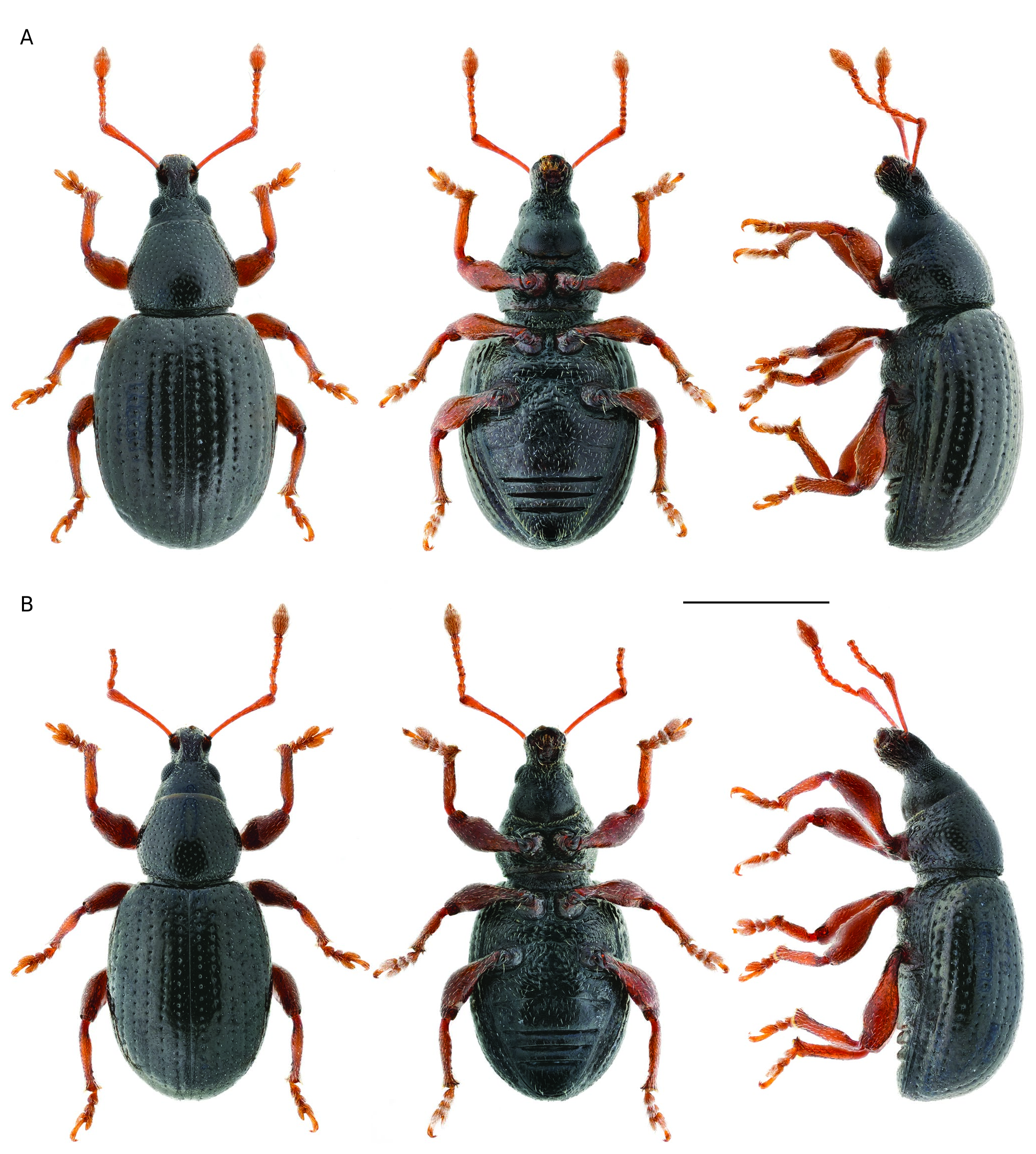
Vestiture ( Figs 2 View FIG ; 3 View FIG )
Body glabrous, with barely visible, sparse, short (15-20 µm), recumbent, piliform setae. Legs and antennae with sparse, recumbent, piliform setae; club tomentose.Tarsal hairy soles well developed.
Colour
Body shiny, dark brown to black, antennae and legs brown to dark brown.
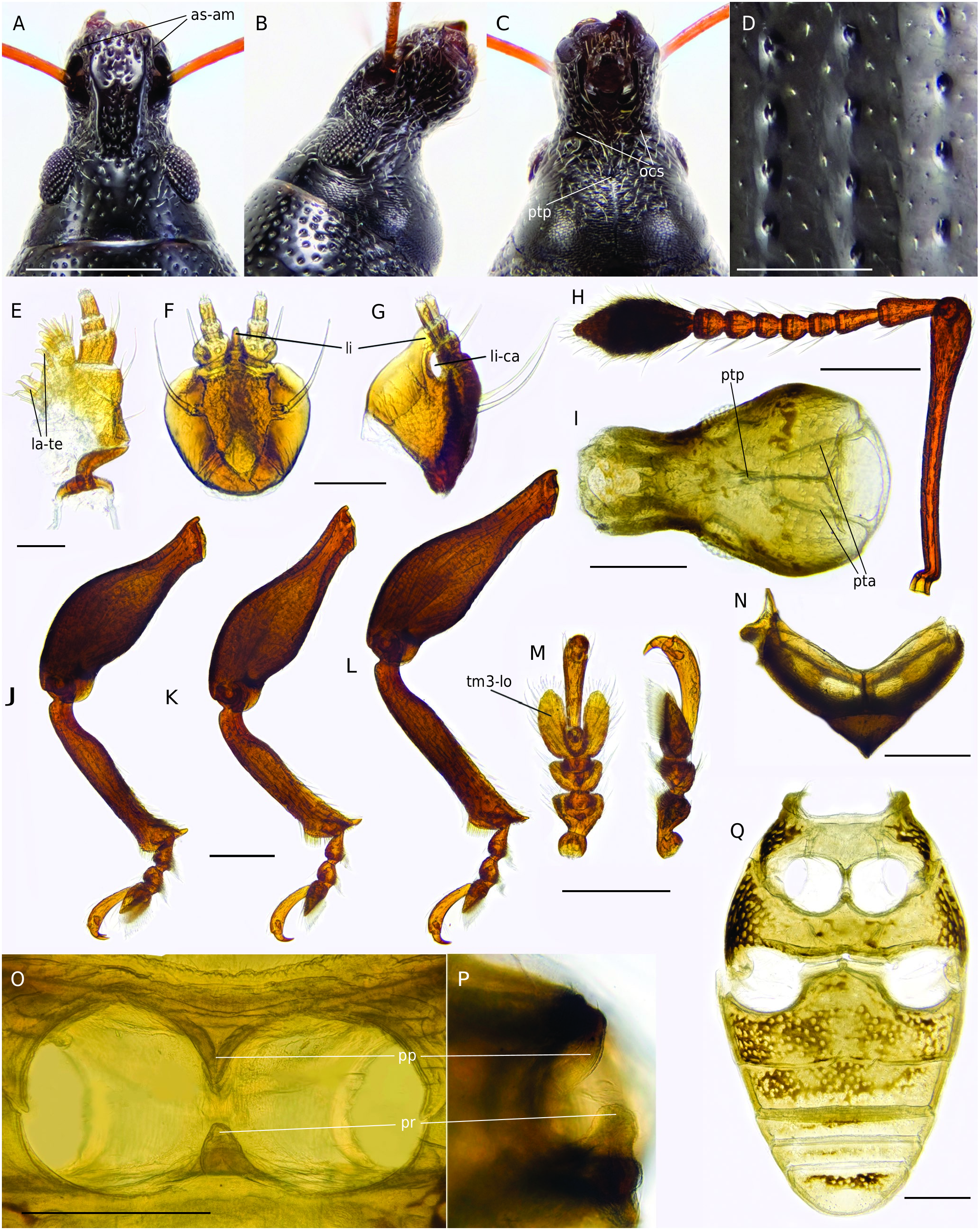
Head ( Fig. 4 View FIG A-C)
Rostrum almost parallel-sided, distinctly abruptly separated from head capsule, not forming a common cone, RL/RW = 0.91-1.17. Pterygia slightly projecting from lateral contour of rostrum. Antennal scrobes dorsal, posteriorly open; dorsal margin of antennal scrobes reaching 2/3 length of rostrum. Epifrons distinctly edged, weakly convergent, at the level of antennal insertion two times wider than at base and 1.5 times narrower than vertex; flat, separated from vertex by weak transversal depression; finely sparsely punctate, interspaces between punctures twice wider than diameter of punctures; median carina and sulcus absent. Frons not separated from epistome by epistomal carina. Vertex flat longitudinally and weakly convex in transversal direction, VW/ELD = 1.13-1.29; median fovea small but distinct. Eyes subdorsal, oval, distinctly convex, contains about 10 ommatidia in ELD. Posterior tentorial pits merged into a single pit ( Fig. 4C, I View FIG ). Hypostomallabial suture convergent medially. Occipital suture transverse.
Antennae ( Fig. 4H View FIG )
Scape evenly curved and widened distally. 1st funicular antennomere distinctly larger than other antennomeres, L/W = 2.36; 2nd funicular antennomere L/W = 1.79; 3rd = 1.08; 4th = 0.95; 5th = 0.88; 6-7th antennomeres L/W = 1; club broadly spindle-shaped, 2.1 times longer than wide, weakly separated from funicle.
Maxilla ( Fig. 4E View FIG )
Lacinia with four long, straight, clustered teeth. Palpiger with two setae. Palpomere 1 quadrate, palpomere 3 L/W = 0.95, with convex sensillar field. Stipes oblong; stipes-palpiger articulation fused.
Labium ( Fig. 4F, G View FIG )
Prementum cordate, with two premental setae; ligula extended beyond anterior margin of prementum, ligula in lateral aspect with cavity, anterior process well sclerotized, rounded, with straight dorsal margin; palpomeres L/W: 1st = 0.68; 2nd = 1.15; 3rd = 1.9. Thorax
Pronotum convex at sides, maximum width posteriad the middle; PL/PW = 0.81-0.9; pronotal disc convex; anterior constriction absent; pronotum at base narrower than base of elytra; punctures piliferous, shallow, narrower than punctures in elytral striae; spaces between punctures equal 1-2 diameter of a puncture. Prosternal process without projection. Prosternellum in ventral aspect forms long process, fused with hypomera, in lateral aspect forms well developed blunt projection ( Fig. 4O, P View FIG ). Mesoventrite and metaventrite with coarse sculpture. Anterior margin of mesonotum concave, prephragmal arms protruded, longitudinal mesothoracic suture well defined ( Fig. 4N View FIG ). Scutellar shield completely reduced.
Elytra
Ovate, weakly convex at sides; EL/EW = 1.21-1.33; disc weakly convex; striae thin, with distinctly edged deep punctures, bearing short, barely visible seta ( Fig. 4D View FIG ); intervals 1.5-2 times as long as puncture diameter; interstriae weakly convex, sparsely punctate with very small punctures bearing short seta, 2.5 times wider than striae. Anterior margin of elytra weakly emarginate.
Legs ( Fig. 4 View FIG J-L)
Femora swollen in middle part, unarmed. Protibia weakly widened at apex, lateral margin almost straight; medial margin entire, sharp, and sinuate in apical half, sparsely pilose; mucro well developed, spur absent. Metatibia widened at apex; medial margin entire, sharp, sinuate in apical half, slightly more densely pilose than mesotibia; mucro well developed, spur absent. Tarsus ( Fig. 4M View FIG ): L/W of 1st tarsomere = 0.75, 2nd = 0.6, 3rd = 1.05, onychium = 4.5; lobes of tarsomere 3 elongate; claws equal, connate at base.
Abdomen ( Fig. 4Q View FIG )
Ventrite 1 with coarse sculpture, intercoxal process obtuse; posterior margin of male ventrite 1 weakly emarginate, in female straight; female ventrite 5 with glabrous field and long piliform setae near apex.
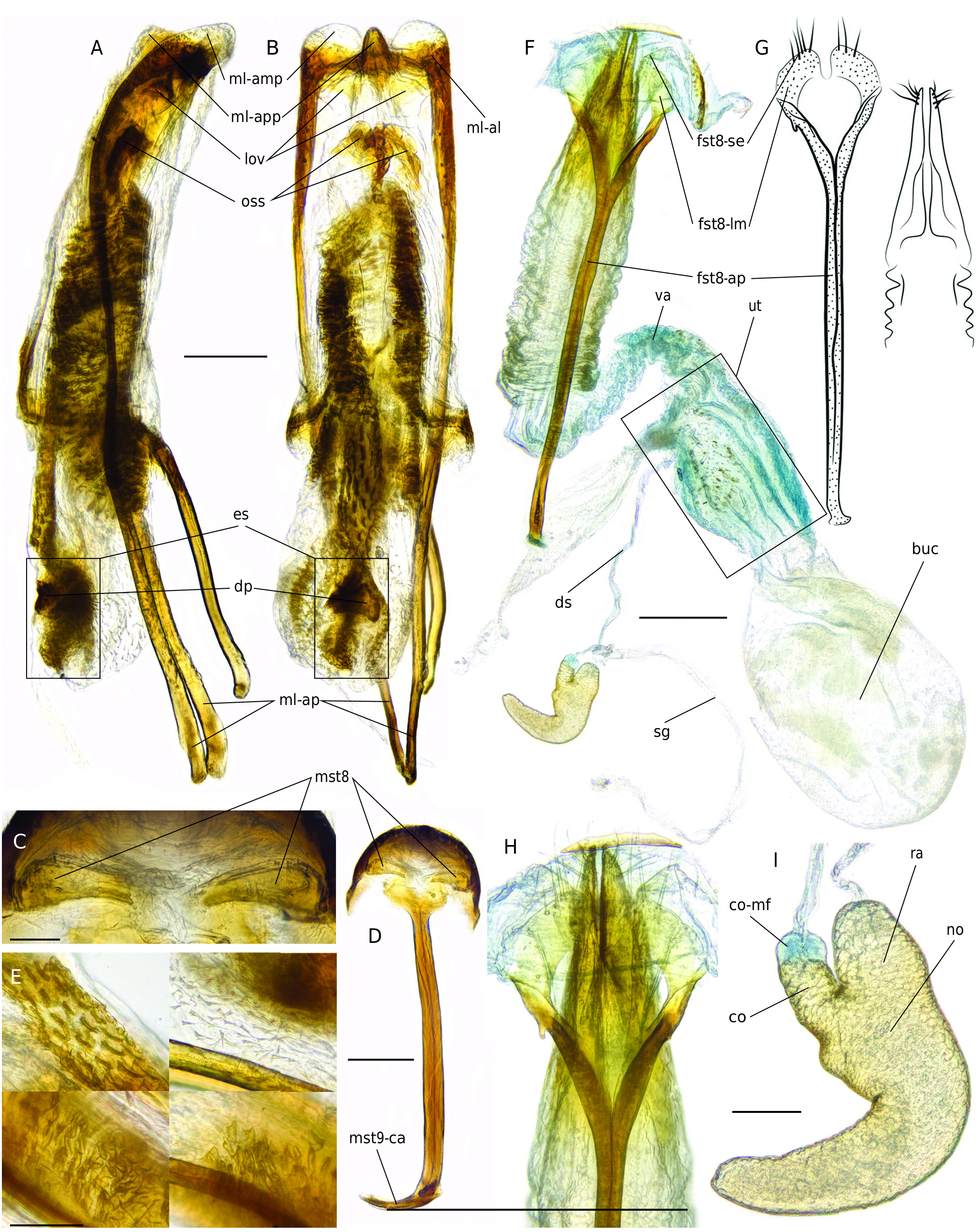
Male terminalia ( Fig. 5 View FIG A-E)
Sternite 8 weakly sclerotized, composed of lanceolate hemisternites. Sternite 9 with lamina weakly sclerotized and divergently forked arms; apodeme thick, straight, with well developed caput. Median lobe parallel-sided, with ventral wall weakly sclerotized; apical preostial process distinct, apical membranous plate and apical lobes well developed; apodemes 2.5 times longer than median lobe. Endophallus semifolded inside median lobe, with lateral ostial valves distinct, weakly sclerotized; armature comprises large unpaired ostial sclerite and large needle-shaped spicules; endophallic sclerite well developed, asymmetrically twisted, flagellar sheath absent, dorsal plate massive. Tegmen with tegminal ring closed; parameres free medially on base, nearly as long as apodeme, apodeme straight.
Female terminalia ( Fig. 5 View FIG F-I)
Ovipositor telescopic, with long sparse setae; styli vestigial. Spermatheca Y-shaped, with thin wall and reticulate sculpture; ramus as long as collum; collum with membranous formation in apical half; velum absent; cornu well developed; nodulus weakly swollen; spermathecal gland narrow, 4.5 times longer than spermatheca; spermathecal duct inserted at uterus. Bursa copulatrix oval, with distal extension without armature. Sternite 8 with apodeme 2.84 times longer than lamina, bifurcate in basal part, margo basalis distinct, caput small; lamina well sclerotized; anterior margin deeply sinuate; setae long, bilaterally aggregated.
Differential diagnosis
In general appearance Humeromima rufipes resembles Omias with defoliated vestiture, and having vestiture most similar to Omias oertzeni Stierlin, 1887 . From most of Omias , it differs in the following characters: body glabrous, with very small (length: 15-20 µm) sparse setae, visible only at highest magnifications; bases of elytra broadly rounded, resembling pseudo humeral calli; rostrum separated from head capsule by transverse depression; epifrons distinctly edged; maxilla with four long, straight, clustered lacinial teeth; tibiae mucronate; endophallus not exceeding length of aedeagal apodemes, with well-developed, needle-shaped spicules of various sizes; spermatheca Y-shaped, ramus as long as collum; apodeme of female 8th sternite strongly forked in basal part, apical margin of lamina deeply emarginate. From Humeromima nitida (Boheman, 1842) it differs by the transverse depression on the rostrum, epifrons distinctly edged, 1st funicular antennomere almost as wide as 2nd; antennal scape less curved and widened distally. From the glabrous species of the genera Bryodaemon and Exomias , H. rufipes differs by the transverse depression of the epifrons, and peculiar structure of aedeagus bearing membranous lobes and preostial process. From Bryodaemon it also differs by having two equal tarsal claws, narrower epifrons and broadly rounded anterior margin of elytra. From Exomias it also differs by having longer rostrum, narrower epifrons, dorsal position of the antennal scrobes, well defined and slightly projecting pterygia.
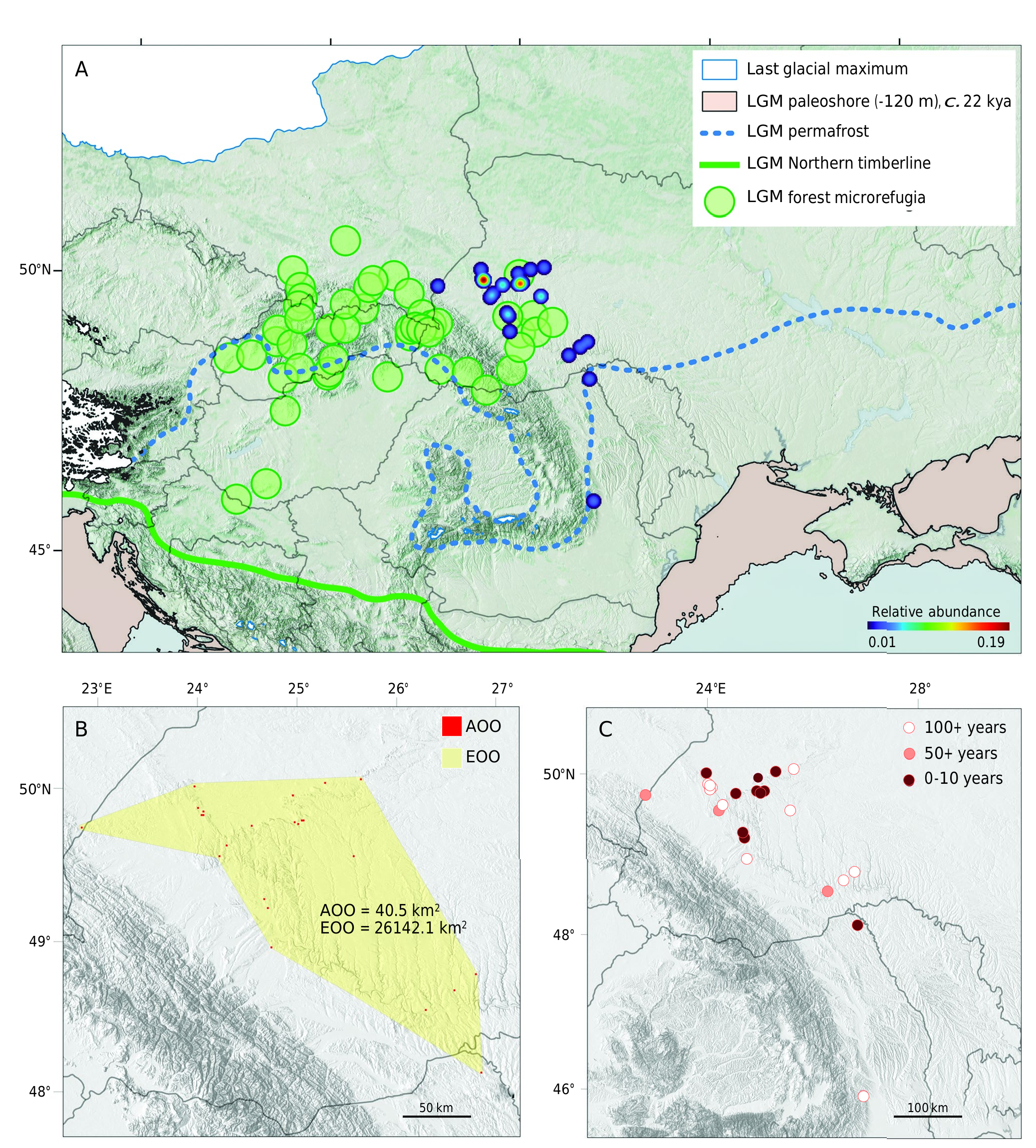
DISTRIBUTION
Poland ( Trella 1930), Ukraine: IFR LWI TER ( Schoenherr 1834; Kuntze & Noskiewicz 1938; Yunakov et al. 2018; Khrapov & Yunakov 2020), Romania ( Liebmann 1920).
HABITAT
H. rufipes occurs mostly in meadow steppes and rock steppes at the hilltops, but maximal abundance is registered in grass steppes.
CO-OCCURRENCE
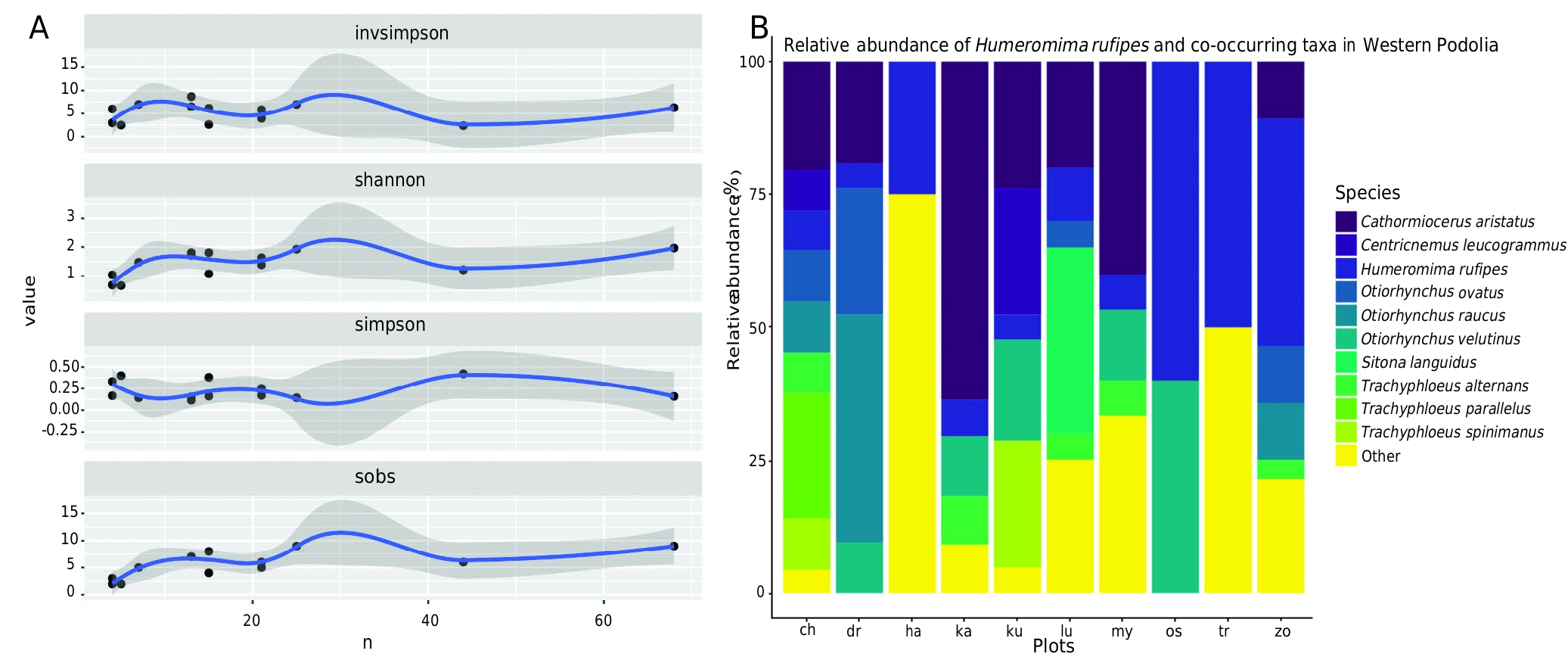
In terms of alpha-diversity the community of Entiminae in the study area is moderately even (Simpson mean= 0.23, Shannon mean = 1.42) and represented by 24 species ( Fig. 7A View FIG ). 87.84% of the community is represented by 10 species arranged by relative abundance as follows: Cathormiocerus aristatus (Gyllenhal, 1827) [27.06], Humeromima rufipes [12.94], Trachyphloeus parallelus Seidlitz, 1868 [8.68], Otiorhynchus raucus (Fabricius, 1777) [8.24], O. ovatus (Linnaeus, 1758) [7.06], O. velutinus Germar, 1823 [5.88], Trachyphloeus alternans Gyllenhal, 1834 [5.49], T. spinimanus Germar, 1823 [5.49], Centricnemus leucogrammus (Germar, 1823) [4.71], Sitona languidus Gyllenhal, 1834 [2.75]. The remainder 17 species share 1.96-0.39% each. Remarkably, the top 10 species co-occurring with subendemic H. rufipes have wide-ranging West Palaearctic or Transpalaearctic distributions ( Fig. 7B View FIG ; Appendices 1; 2).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Entiminae |
|
Tribe |
Omiini |
|
Genus |
Humeromima rufipes (Boheman, 1834)
| Khrapov, Denys & Yunakov, Nikolai 2024 |
Humeromima rufipes
| KHRAPOV D. & YUNAKOV N. 2020: 144 |
| YUNAKOV N. & NAZARENKO V. & FILIMONOV R. & VOLOVNIK S. 2018: 273 |
| BOROVEC R. 2013: 298 |
| PODLUSSANY A. 1998: 81 |
Omiamima rufipes
| MAZUR M. 2002: 230 |
| DIECKMANN L. 1980: 197 |
| SILFVERBERG H. 1977: 227 |
Omias rufulipes
| MARSEUL S. A. & DE 1873: 562 |
Omias rufipes
| SMRECZYNSKI S. 1966: 49 |
| KUNTZE R. & NOSKIEWICZ J. 1938: 345 |
| TRELLA T. 1930: 7 |
| RYBINSKI M. 1901: 141 |
| SCHOENHERR C. J. 1834: 500 |