Xylosandrus crassiusculus (Motschulsky), 2016
|
publication ID |
https://doi.org/10.11646/zootaxa.4877.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:3CABEE0D-D1D2-4150-983C-8F8FE2438953 |
|
DOI |
https://doi.org/10.5281/zenodo.4424271 |
|
persistent identifier |
https://treatment.plazi.org/id/2127217C-C846-DC1A-FF44-F028EEABBF74 |
|
treatment provided by |
Plazi |
|
scientific name |
Xylosandrus crassiusculus (Motschulsky) |
| status |
|
- Xylosandrus crassiusculus (Motschulsky) View in CoL
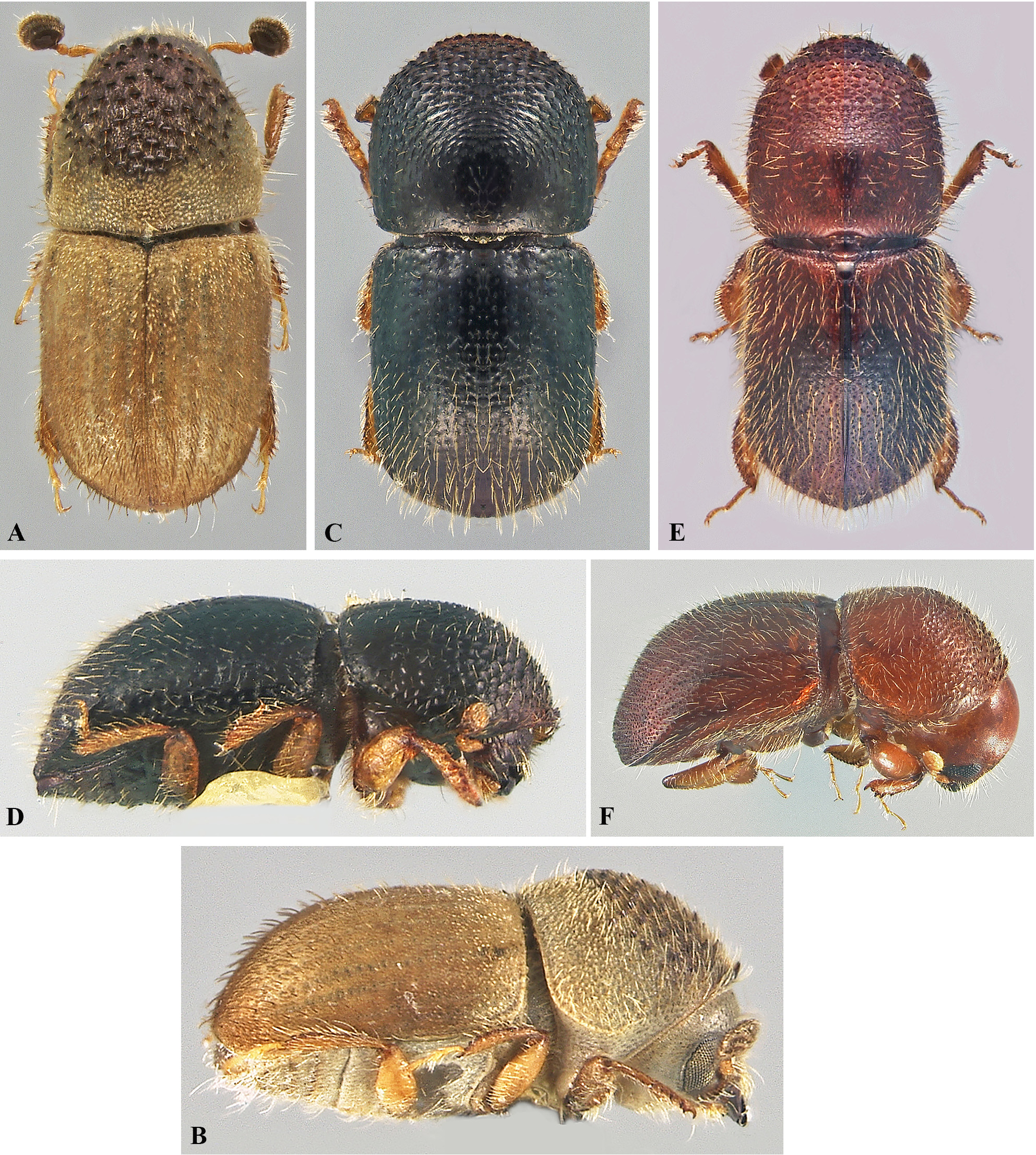
( Figs. 3E, 3F View FIGURE 3 )
Distribution. Native to Asia, X. crassiusculus is a cosmopolitan species, present on all continents. In Europe, it was detected for the first time in Italy in 2003 in Tuscany ( Pennacchio et al. 2003) and then spread across the country in the regions of Liguria, Veneto, Friuli Venezia Giulia, Lazio, Campania and Piedmont ( Gallego et al. 2017). In France the species was repeatedly intercepted in the port of Le Havre, Seine-Maritime, on wooden packaging materials from China, and then recorded in southeastern France in 2014 ( Nageleisen et al. 2015), in Switzerland in 2015 ( Daubrée 2016), in Spain in 2016 in the Valencia region ( Gallego et al. 2017), and finally in Slovenia in 2017 ( Kavčič 2018). The distribution of X. crassiusculus in France was relatively restricted until 2017, confined to east of the Alpes-Maritimes, mainly in the town of Nice in the Mont-Boron forest. At the margins of this infestation, the species was trapped in four municipalities of the same district. Despite the eradication attempts set up in 2014 for the Nice infestation, the species is now established and clearly expanding in France ( Daubrée 2016). Since 2018, two new breeding populations have been discovered in south-west of France, far from the first introduction sites ( Roques et al. 2019). As with Xylosandrus germanus ( Nageleisen et al. 2015) , X. crassiusculus seems able to quickly colonize new areas and could spread over a large part of France in only few decades.
New records: ALPES-MARITIMES – Biot, Vaugrenier Park, interception trap, 2016, 1 ind., Y. Braud leg. ; Cannes , Sainte Marguerite Island, intercepting trap baited with ethanol 20%: 36 ind. from 01.IV. to 08.VII.2014, 23 ind. from 14.IV. to 21 VII.2015 and 37 ind. from 12.IV. to 05.VII.2016; ibidem, interception traps baited with ethanol 100%, (-) α-pinene, and a pheromone blend for longhorn beetles, from 24. V. to 27.IX.2019, 13 ind., URZF leg.; Menton , interception traps baited with ethanol 100%, (-) α-pinene, and a pheromonal blend for longhorn beetles, from 24. V. to 27.IX.2019, 163 ind., URZF leg.; Nice , bottle trap and interception trap baited with ethanol 20%: 1,760 ind. in 2015, 5,254 ind. in 2016, 23,360 ind. in 2017 and 89 ind. in 2018; ibidem, interception traps baited with ethanol 100%, (-) α-pinene, and a pheromonal blend for longhorn beetles, 2018, 735 ind ., URZF leg.; Saint-JeanCap-Ferrat , bottle trap and intercept trap initiated 20%: 1 ind. in 2016 and 15 ind. in 2017 , DSF leg.; La Turbie , trap bottle and trap intercept baited with ethanol 20%, 2018, 5 ind ., DSF leg.; ibidem, interception traps primed ethanol 100%, (-) α-pinene, and a pheromonal blend for longhorn beetles, from 06.VII. to 27.VIII.2018, 33 ind ., URZF leg.; Villefranche-sur-Mer , sight on Judas tree, 17.X.2016, 28 ind., E. Chapin leg. ; Villeneuve-Loubet , interception traps baited with ethanol 100%, (-) α-pinene, and a pheromonal blend for longhorn beetles, from 16.VII. to 27.VIII.2018, 10 ind ., URZF leg.; HERAULT – Gignac , interception traps baited with ethanol 100%, (-) α-pinene, and a phero-mone blend for longhorn beetles, from 24. V. to 27.IX.2019, 13 ind., URZF leg.; LANDES – Saint-Maurice-surAdour , VIII.2019, massive attack on a Crape myrtle, C. Le Bihan leg. ; PYRENEES-ATLANTIQUES – Guiche , deciduous forest, intercepting trap, from 24.VII to 18.IX.2018, 151 ind., N. Komeza leg. ; SEINE-MARITIME – Le Havre , port area, caught on wooden packaging material, 25.VII.2013, 35 ind., CEP Le Havre leg. ; ibidem, 29.VII.2014, 2 ind., CEP Le Havre leg.; ibidem, 28.VII.2016, 1 ind., CEP Le Havre leg.
Biology and ecology. X. crassiusculus is a very polyphagous species infesting both hardwoods and conifers. It is known from 124 plant species (mainly tropical) belonging to 46 families ( Schedl 1963). It has been observed also on fruit ( Prunus, Malus , Ficus , etc.), forest ( Alnus , Populus , Salix , Quercus , etc.) and ornamental ( Acacia, Hibiscus, Magnolia , etc.) tree species. In France, this insect has been detected on carob tree ( Ceratonia siliqua L.), Judas tree ( Cercis siliquastrum L.), olive trees ( Olea europea L.) and crape myrtle ( Lagerstroemia indica L.). In the Mediterranean region, X. crassiusculus seems to show a clear preference for the carob tree in France, Italy and Spain ( Pennacchio et al. 2003; Gallego et al. 2017). However, in the southwest of France (Guiche), many specimens were trapped in a deciduous forest dominated by oaks, showing the species able to reproduce in native tree species other than carob. X. crassiusculus is an ambrosia beetle infesting both stressed and recently dead trees, although colonization of apparently healthy host plant is not unusual ( Atkinson et al. 2011). Females can colonize twigs, branches and small trunks ( 2 to 30 cm in diameter) with preference for small branches and stems ( Atkinson et al. 2011).
Damage and infestation risk. Damage caused by X. crassiusculus is mainly related to the activity of females boring deep galleries into the sapwood. However, like all ambrosia beetles, X. crassiusculus can be a vector of phytopathogenic fungi that could play a role in the host dieback. Aside from the leaf drying on the infected twigs, the most noticeable infestation symptom in carob trees and some other host species is the presence of white sawdust cylinders expelled by females from the entrance holes ( Atkinson et al. 2011; Daubrée 2016). However, this symptom is observable only during the first days of the stem colonization, making later detection of this species more difficult ( Daubrée 2016). Massive colonization leads to dieback and death of the infected trees ( Atkinson et al. 2011; Daubrée 2016). Massive attacks lead to the death of trees. Smith & Hulcr (2015) describe instances of the species attacking living trees in water-stressed conditions (flooding root system). The damage related to X. crassiusculus in France has for the moment a mainly environmental relevance, as carob tree is a plant species protected at national level and carob forests constitute a habitat of community interest under the Fauna Flora Habitats Directive (European Directive 92/43/CEE of 21 May 1992).
| URZF |
Institut National de la Recherche Agronomique - Orleans. Unite de Zoologie Forestiere |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scolytinae |
|
Genus |