Gonatodes infernalis, Rivas, Gilson A. & Schargel, Walter E., 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.184780 |
|
DOI |
https://doi.org/10.5281/zenodo.6228158 |
|
persistent identifier |
https://treatment.plazi.org/id/206F8788-FF87-FFD4-FF24-CDB0548DF087 |
|
treatment provided by |
Plazi |
|
scientific name |
Gonatodes infernalis |
| status |
sp. nov. |
Gonatodes infernalis , sp. nov.
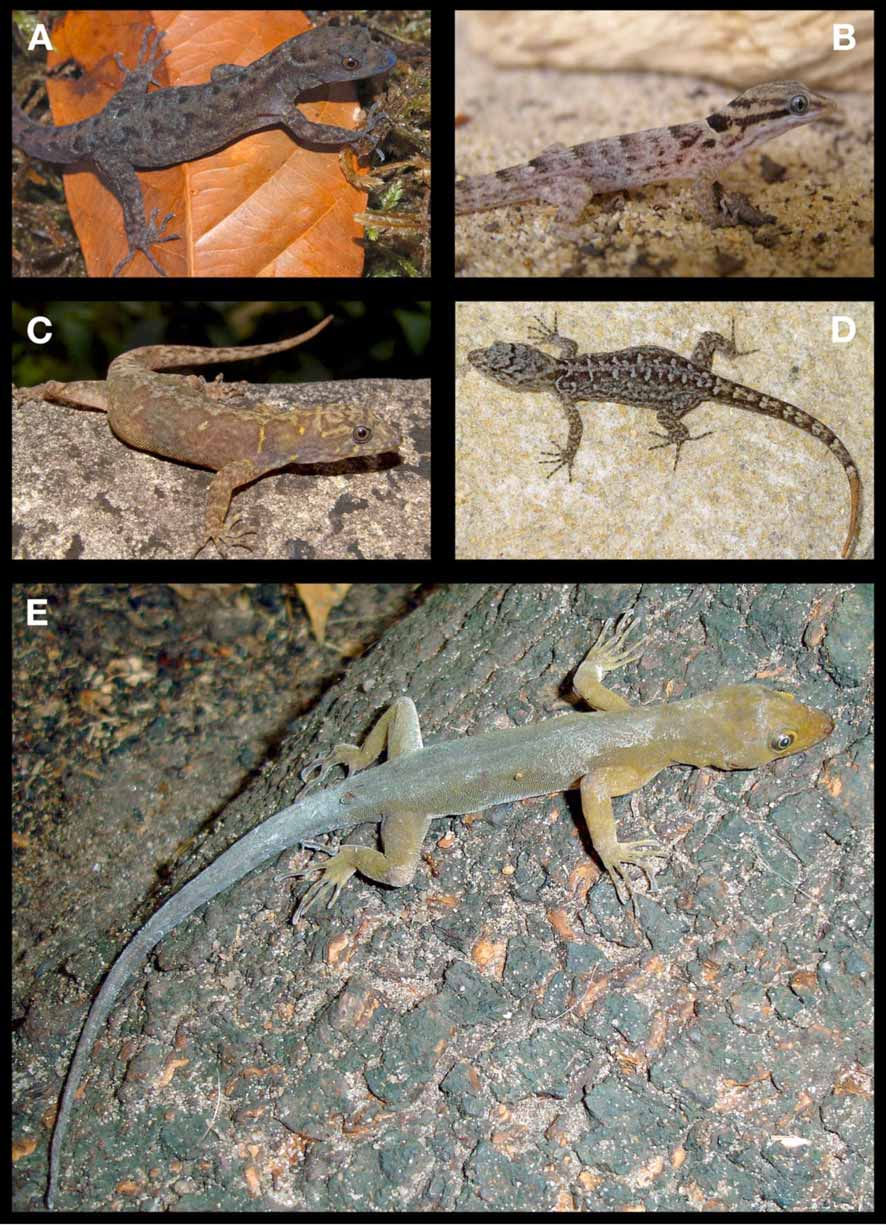
Fig. 1 View FIGURE 1 e.
Holotype. MHNLS 18440 (field number CJF4703), an adult female, collected in Sector El Infierno, on road Puerto Ayacucho-Gavilán, approximately 100 m asl. Estado Amazonas, Venezuela: one of two specimens collected on 15 March 2007 by Gilson Rivas and Tito Barros.
Paratypes. All females: MHNLS 18439, specimen with the same collection data of the holotype; UTA R- 55378–79, MHNLS 18397, three specimens from the same locality, obtained by Gilson Rivas, Coleman Sheehy III, Carl Franklin, and Tito Barros on 14 March 2007.
Diagnosis. The new species can be distinguished from all congeners by a combination of very large size, supraciliary spine absent, females with immaculate brown dorsal coloration, and a subcaudal scalation pattern (only in the unregenerated tail) of a single medial row of wide scales which laterally contact either two or three adjacent caudal scales in a 1:1 alternating fashion (See Fig. 2 View FIGURE 2 ). Gonatodes infernalis is, to our knowledge, the largest species of sphaerodactyl gecko, with adult females reaching nearly 65 mm in SVL, which readily separates this species from the much smaller G. albogularis , G. antillensis , G. atricucullaris , G. daudini , caudiscutatus, G. eladioi , G. humeralis , G. petersi , G. tapajonicus and G. vittatus , none of which exceed 45 mm in SVL (data from: Avila-Pires 1995; Rivero-Blanco 1979). The new species is also unique in the genus in having females with a coloration devoid of any well defined markings, a character state that separates it from all other species of comparable size (and also from the smaller species), namely G. alexandermendesi , G. annularis , G. ceciliae , G. concinnatus , G. falconensis , G. hasemani , G. ocellatus , G. purpurogularis , G. seigliei and G. taniae , all of which have females with well defined and conspicous dark markings on the head and the body. Among Amazonian/Guianan species, G. infernalis further differs from G. alexandemendesi and G. hasemani in lacking an elongated supraciliary spine. Finally, G. infernalis differs from all other species of Gonatodes , except G. eladioi (a much smaller species) in the subcaudal pattern of the unregenrated tail (described above). All other species of Gonatodes have subcaudal pattern (for a description of the different character states and taxonomic distribution see Rivero-Blanco 1979) in which one of the following states occur: a) the medial scales that contact three scales laterally occur every two medial scales that are in contact with two scales laterally, b) with a divided medial scale every two single medial scales, c) proximally with a pattern as described in G. infernalis , but switching to the pattern described in “b” distally, or c) with medial scales not differentiated from adjacent lateral scales.
Description of Holotype. an adult female, with snout-vent length of 56.2 mm. Tail length 74.5 mm, complete and not regenerated. Head 1.5 times longer than wide (HL: 14.5 mm; HW: 10.0 mm). Snout 4.1 mm long, acutely rounded in dorsal view, gently sloping toward top of head. Neck slightly narrower than head and body. Body nearly cylindrical but wider than high; axilla-groin distance 24.3 mm. Limbs well developed with relatively long digits, fourth toe length 9.1 mm, 0.85 times shank length (10.8 mm). Tail round in cross section, tapering toward tip.
Tongue relatively wide with bluntly rounded tip, covered by scalelike papillae, which become smaller posteriorly; tip of tongue with a short median cleft. Teeth small, subequal, conical.
Rostral large, with two small, closely spaced vertexes on upper margin, visible from above, with a median cleft extending forward from posterior margin to near tip of snout. Three postrostrals, lateral ones (supranasals) distinctly larger than medial, medial postrostral slightly larger than adjacent scales between anterior margin of the orbit and the postrostrals. Nostril bordered by rostral, three postnasals, lateral postrostral (supranasal), and in point contact with first supralabial. Postnasals slightly larger than adjacent loreals. Scales on snout and on loreal region roughly round, granular, juxtaposed, but somewhat conical posteriorly. Loreal scales number about 15 (both sides) in a line between postnasals and anterior margin of orbit. Scales decrease slightly in size from the postrostrals toward posterior part of head. Scales on supraorbital region similar (in size and shape) to and continuous with those on top of head. Supraciliary flap not developed and without an elongate supraciliary spine, supraciliary scales conical, somewhat larger and protruding laterally on anterior half of row. Pupil round. Supralabials 5 (both sides), first largest, second through fifth roughly subequal, anterior portion of fifth scale below center of eye. Scales on temporal region similar to those on posterior top of head. Ear opening (1.3 mm) much smaller than eye (3.0 mm), obliquely oval.
Mental large, with round anterior margin following lower lip, posteriorly with three crooked edges of which the laterals run obliquely posteromedially from lip to vertex with the medial transverse edge. Postmentals 2, distinctly larger than adjacent posterior scales. Scales on chin small and polygonal directly behind postmentals, granular and tiny posteriorly; a few larger, polygonal scales adjacent to infralabials, juxtaposed; Infralabials 7 (both sides), decreasing in size posteriorly, first two very large and projecting onto chin.
Scales on nape and on sides of neck granular, continuous with those on head and body. Scales on throat smooth, imbricate, with round posterior margin, with a short transitional area with the granular scales on chin and gular area.
Dorsals granular, on the vertebral area similar in size to scales on snout; dorsolaterally and on flanks slightly larger. Transition between scales on flanks and ventrals somewhat abrupt but not clearly demarcated. Ventral region with scales distinctly larger than dorsals, slightly smaller on chest than on belly, smooth, with round posterior margin, imbricate (each scale overlapping anterior portion of scale lying posteriorly); ventrals in oblique rows, on belly also forming rather regular longitudinal rows, with 56 scales along the midventral line between anterior margin of forelimbs and vent. Scales around midbody about 115, of which 20 are ventrals. Scales on preanal plate similar to ventrals, excepting border of vent, which has minute scales arranged in three rows. Without escutcheon area on abdomen.
Scales on dorsum of tail base abruptly become flat, smooth, rounded in shape, imbricate (rather than conical), just posterior to level of vent. Underside of tail with smooth, flat, imbricate scales, increasing in size toward midventral line; first 8 small subcaudals posterior to vent on midventral row increasing in size posteriorly but not clearly differentiated from adjacent laterals, followed by a single longitudinal row of significantly enlarged, roughly hexagonal, medial subcaudals; each medial subcaudals wider than long with anterior and posterior margins parallel and in transverse position, lateral two margins converging distally forming a somewhat sharp vertex, lateral margins contacting two or three adjacent scales laterally in an alternating fashion.
Scales on limbs granular and juxtaposed on dorsal and posterior surfaces, otherwise scales are smooth, flat, roundish, imbricate. Lamellae under first (I) through fifth (V) finger (infraproximals in parentheses): I: 11/11(2/2), II: 17/16(5/5), III:?/20(6/7), IV: 22/21(8/8), and V: 18/17(6/6), respectively. Lamellae under first through fifth toe (infraproximals in parentheses): I: 12/11(3/2), II: 18/17(6/5), III: 21/20(6/6), IV: 25/24(10/ 10), and V: 19/19(5/5), respectively. Fingers and toes with four lateral rows of scales distally, with occasional reduction to three rows in some sections, especially near the claw. Claws exposed, non-retractile, between two basal scales (1 dorsal, 1 ventral).
Color in preservative. Dorsum and sides of body and tail uniform grayish brown with top of head and limbs slightly paler. Microscopically each dorsal scale is cream-colored with many evenly spaced, welldefined melanophores. Interstitial dorsal skin more densely covered with melanophores than individual scales. Venter pale grayish brown, becoming paler towards vent and pectoral region. Microscopically the ventral scales are cream-colored with evenly spaced melanophores throughout most of the venter, but towards the vent the melanophores are found only bordering the posterior margin of each scale which accounts for the change in color saturation described above. In the pectoral and gular region the melanophores occur at smaller densities per scale. Ventral surface of tail slightly paler than dorsal surface. Anterior half of tongue dark gray, posterior half white.
Variation in paratypes. The paratypes are four adult females ranging in SVL from 57.2 to 65.5 mm. Both supralabial and infralabial counts vary from 5 to 7. The paratypes have 3 postrostral as in the holotype, except MHNLS 18439, which has four (two medial small scales between large paired supranasals). Scales around the midbody range from 95 to 105 in three specimens (not counted in MHNLS 18439 due to body skin loss) of which 20–22 are ventrals. There are 58–60 ventral scales along the trunk. The variation in the number of lamellae under first through fifth fingers: I: 10–12(2–3), II: 17–22(5–6), III: 21–24(7–9), IV: 21–24(7–9), and V: 16–18(5–6). The variation in the number of lamellae under the first through fifth toe: I: 10–13(2), II: 16–19(5–7), III: 21–25(6–8), IV: 23–28(10–12), and V: 19–20(5–6). The regenerated tail (described on UTA R-55378) has a subcaudal pattern that differs significantly from the original tail. In the regenerated tail the subcaudal scales form a single row of roughly rectangular, wide, but very short plates (longest side is transversal to longitudinal axis of tail), each of which extends laterally to the ventrolateral surface of tail. These plates come into contact laterally with small scales that look just like those in the original tail. Morphometric variation of the type series is presented on Table 1.
The coloration in preservative in the paratypes is essentially as in the holotype with only minor variations in color saturation owing to differences in melanophore densities. Two specimens, MHNLS 18373 and 18397, are noticeably darker compared to the rest of the type series, and especially on the venter which is not as distinctly paler than the dorsum as in the other specimens. The coloration in life was essentially the same as in preservative with the few exceptions noted below. In life all specimens were dull brown dorsally and had a vaguely distinct, yellow coloration suffused on the top of the head that was lost in preservative. The eyes has black pupils, narrowly edged by a yellow iris, and a brown sclera.
Table 1. Morphometric variation in the type series of Gonatodes infernalis .
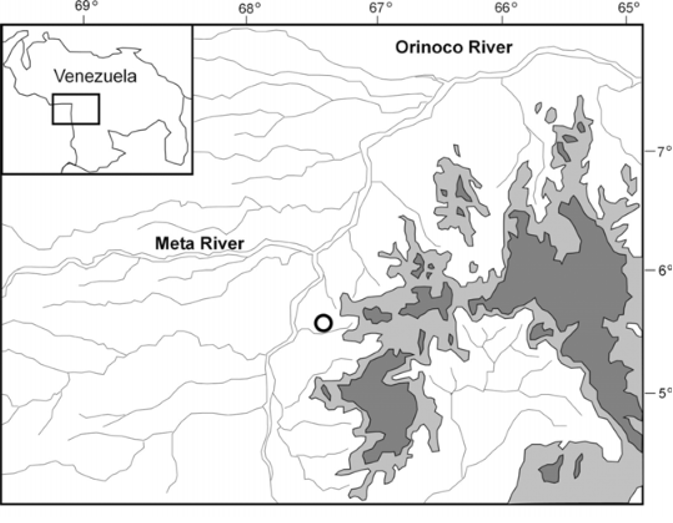
Distribution and Natural History. Known only from the type locality near the city of Puerto Ayacucho, Amazonas, Venezuela ( Fig. 3 View FIGURE 3 ). All the specimens were collected on large, dark, granitic, isolated inselbergs that stand out abruptly from the surrounding plains, and which are locally known as “lajas”. Specimens were collected directly from the rock walls of two nearby and relatively small inselbergs. The area where the specimens were collected was recently burned by indigenous people of the Piaroa tribe, with the purpose of preparing the land for agricultural use in the following rainy season. Three of the specimens were collected between 19:00-20:00, from an inselberg consisting of three large rocks placed in such a way that they formed a small “C” shaped refuge on the ground, which protected the enclosed area from fire and also helped retain water in it ( Fig. 4 View FIGURE 4 ). In this small “oasis” of about 40 m 2 several specimens of Dendrobates leucomelas and Pristimantis sp. were found, in addition to two Bothrops atrox . The two other specimens were collected at about 18:00 on the top inner surface of the entrance of a cave-like, horizontal hole on a nearby inselberg. The hole was located about 40 cm above the ground, had an opening of about 30 cm in diameter and was more than 1 m deep. In the same area two Bothrops atrox were observed at the entrance of holes at the base of inselbergs. Because different animals were observed dead or agonizing (including two snakes in the genus Chironius ) in the recently burned, surrounding area, the above observations indicate that inselbergs may act as refuges that protect animals from fire.
Etymology. The name infernalis is a Latin adjective (masculine & feminine) meaning infernal or hellish. The name was given in reference to sector “El Infierno” (The Hell), the locality where the type series was collected, an area that had been burned recently.
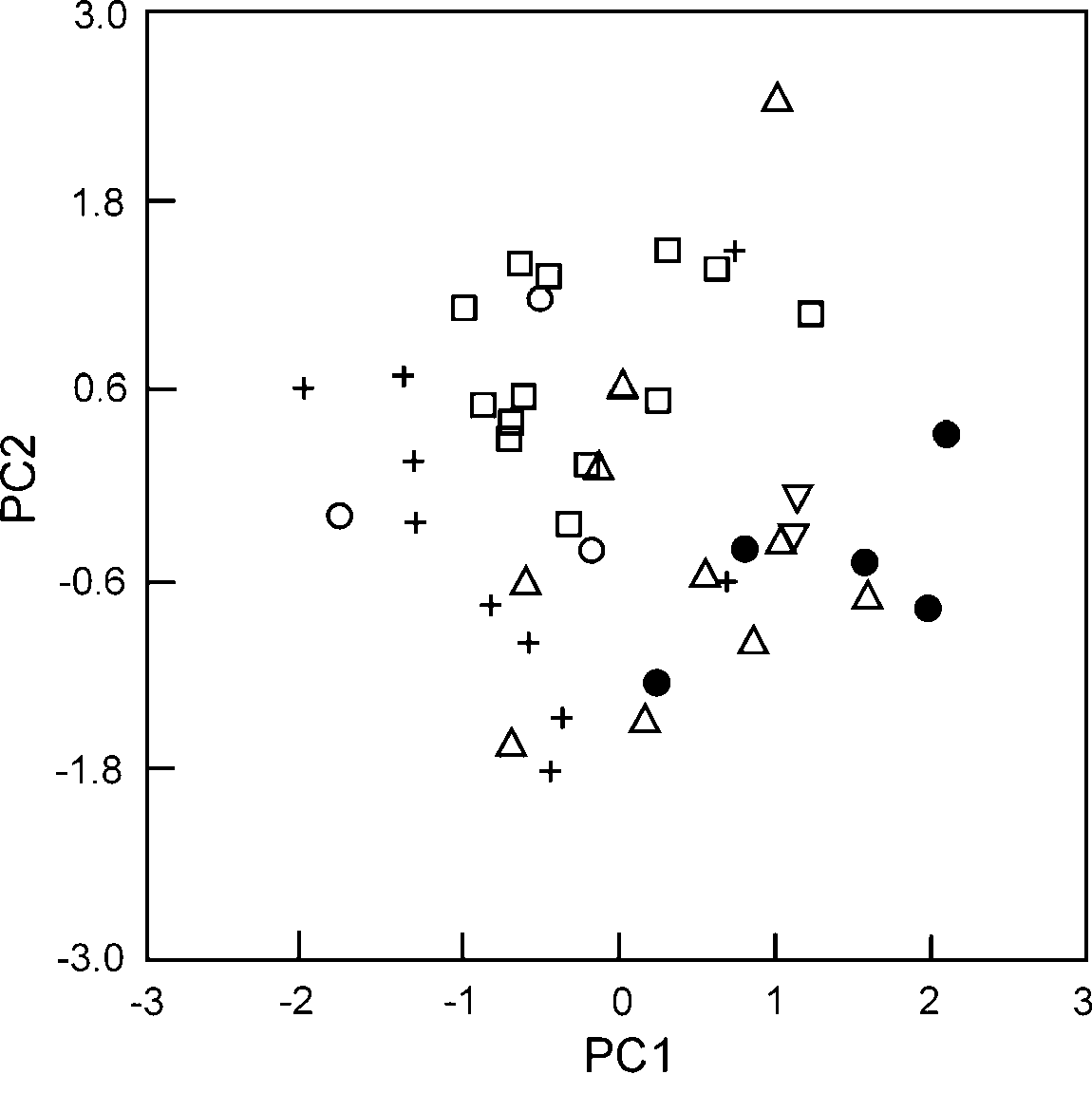
Statistical analysis. Only two principal components (PC) were retained with eigenvalues higher than one. These first two PC (PC1 and PC2) explained 51.1% and 16.5% of the variance, respectively. Component loadings were high for all limb elements on PC1 whereas HDP was the only variable with high loadings on PC2 (see appendix 2). Gonatodes infernalis had, on average, the highest factor scores on PC1 ( Fig. 5 View FIGURE 5 ), indicating that this species in general has relatively longer limb elements compared to the other species included in the analysis. Although there are species-specific differences in PC2 (e.g. between G. infernalis and G. annularis ), G. infernalis does not seem to be particularly distinct within the genus in terms of HDP.
Discussion. The few known specimens of Gonatodes infernalis were collected from inselbergs in the type locality, whereas two other species of Gonatodes ( G. humeralis and G. sp., Schargel et al. in prep) were found in the surrounding forested areas. There are two morphological features in G. infernalis that might represent adaptations to life on inselbergs. The first is the unique uniform, background-matching, coloration in this species, which might confer better crypsis than a color pattern with markings (as in all other species of Gonatodes ), when contrasted to the generally uniformly colored rock surface of the Inselbergs that they inhabit. The other trait, as evidenced on the PCA, is relatively longer limb elements when compared to other congeners. Revell et al. (2007) suggested that longer limbs in rock-dwelling species would be beneficial to prevent individuals from rolling along the long axis of the body and would also confer greater sprint speed while maintaining the body close to the locomotion surface. Furthermore, based on this functional explanation Revell et al. (2007) predicted that selection on limb elongation in rock-dwelling species would be strongest on the femur. The high component loading for FEM (0.833) on PC1 is consistent with Revell et al. (2007) prediction, yet FTL (component loading on PC1=0.858) accounted for most of the variation observed in size-corrected limb element measurements. Interestingly, the first thing we noticed upon examination of G. infernalis was the long digits (quantified in the analysis with FFL and FTL) of this species compared to other Gonatodes . Revell et al. (2007, see also Vitt et al. 1997) also indicated that some rock-dwelling lizards have exceptionally flatter heads and bodies as this would allow them to retreat to narrow crevices to avoid predator attacks. However, this does not seem to be the case with G. infernalis as it does not differ significantly from other congeners in relative HDP. This result is actually not unexpected, as specimens of G. infernalis were not found associated with crevices but with large holes that result from a characteristic erosion phenomenon known as “pseudocarst”, which creates a series of channels, gullies and depressions on these rock formations ( Gröger 2000).
If we assume that the above observations do indeed indicate that Gonatodes infernalis is specialized to live on inselbergs, it is logical to expect that the distribution of this species is limited in part by the distribution of these particular rock formations. Inselbergs are common physiographic elements throughout the peripheral lowlands of the Guiana Shield, from French Guiana in the east to southeastern Colombia on the west ( Gröger 2000). However, Gröger (2000) found that there is hardly any inselberg-endemic species of plant occurring in both the western ( Colombia, Venezuela) and the eastern part ( French Guiana, Suriname) of the Guiana Shield, and indicated that this is probably the result of past climatological events such as a Pleistocene dry belt crossing the Guiana Shield diagonally. Interestingly, the type locality of G. infernalis falls in a region that represents a center of endemism and diversity (the “Atures” center following Gröger 2000) of inselberg associated plant species within the western peripheral lowlands of the Guiana Shield. This Atures center of endemism occurs at the intersection of two phytogeographic units that were defined based on different inselbergendemic species that extend in distribution to either north or south from the Atures center. This pattern seems to result from contrasting climatological conditions, especially precipitation, north (dry) and south (humid) from the Atures center. Based on these observations we believe that G. infernalis is restricted to the western peripheral lowlands of the Guiana Shield, and within this region, it is possible that this species follows one of the patterns exhibited by plant species associated to Inselbergs (e.g., endemic to the Atures center, or extending either north or south from there).
Although males of Gonatodes infernalis are yet to be collected we speculate about their coloration based on some features observed in specimens available to us and the fact that some of the dark and pale markings found in females of other species of Gonatodes seem homologous (based on topographical similarity) with the distinct coloration ornaments found in males. As mentioned above, G. infernalis is unique in the genus in having females with uniform body coloration devoid of any markings; thus, it is possible that males of G. infernalis are not as sexually dichromatic as most other species in the genus. However, the fact that in life all specimens of G. infernalis had more of a yellowish hue on the head relative to the body might be an indication that males have a distinct yellow hood such as in many other species of Gonatodes . Collecting and studying male specimens should be the immediate focus of future studies of G. infernalis , as this would potentially shed lights on the relationships of this species to other members of this genus as well as revealing how sexual dichromatism has been affected by selection towards cryptic coloration in a novel environment.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.