Clytia cf. gracilis (M. Sars, 1850 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4808.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:64E656F6-FBD7-4BA2-B399-B10A97CBEF72 |
|
DOI |
https://doi.org/10.5281/zenodo.6301351 |
|
persistent identifier |
https://treatment.plazi.org/id/1A5A002B-FFE2-6D64-28E3-3E83836952B8 |
|
treatment provided by |
Plazi (2020-07-02 08:10:11, last updated 2023-10-31 18:31:20) |
|
scientific name |
Clytia cf. gracilis (M. Sars, 1850 ) |
| status |
|
Clytia cf. gracilis (M. Sars, 1850) View in CoL
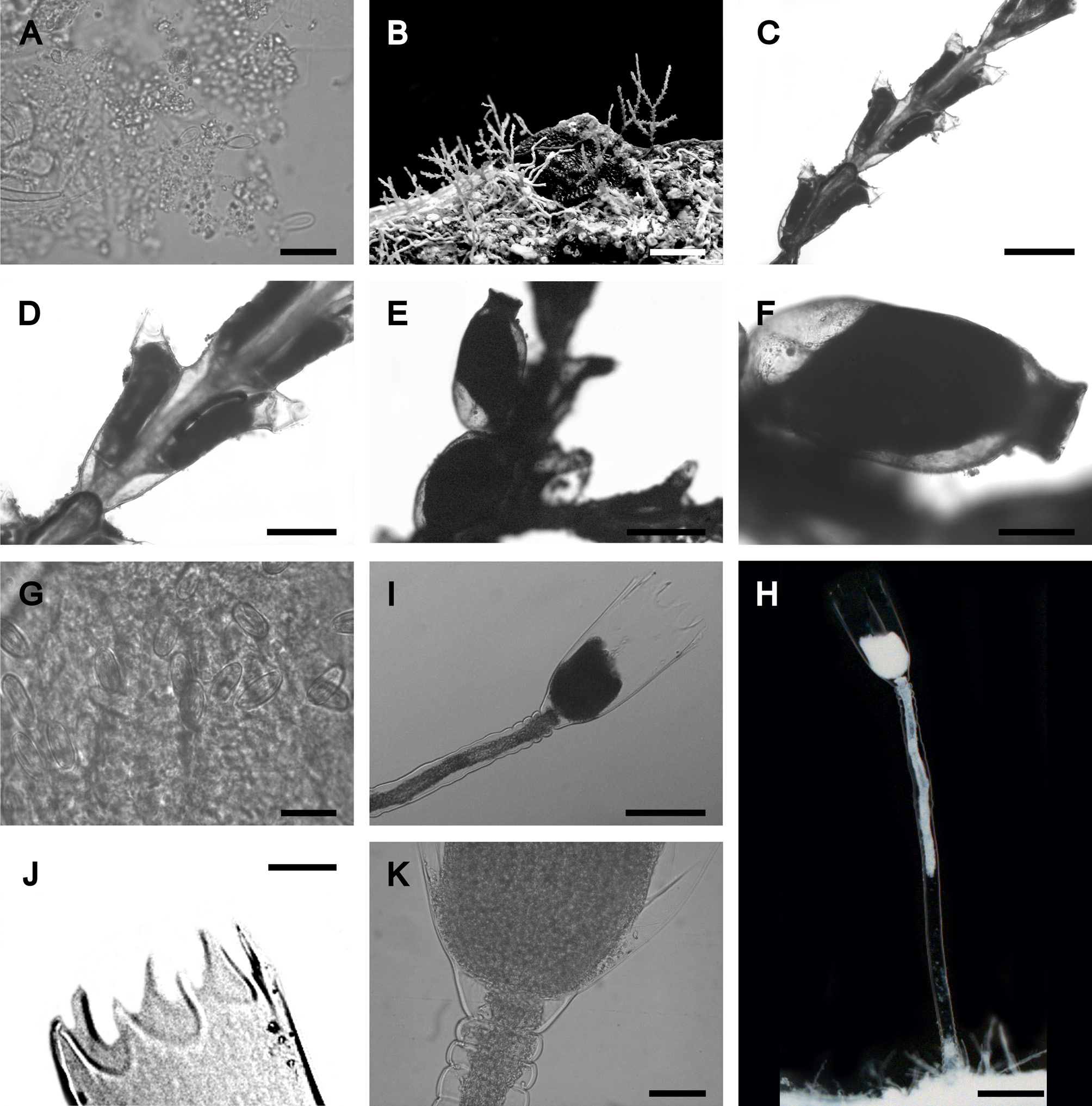
Figs. 7 View FIGURE 7 H–K
Laomedea gracilis M. Sars 1850: 138 View in CoL .
Clytia cylindrica L. Agassiz 1862: 306 View in CoL , figs. 41–44.— Fraser 1938a: 30; Fraser 1948: 206.
Campanularia attenuata Calkins 1899: 350 View in CoL , pl. 2, figs. 9, 9a, 9b, 9c, pl. 6, fig. 9d.
Gonothyraea gracilis View in CoL .— Fraser 1938a: 35; Fraser 1938b: 109; Fraser 1938c: 132; Fraser 1948: 212.
Clytia attenuata View in CoL .— Salcedo-Martínez et al. 1988: 13.
Clytia gracilis View in CoL .— Bastida-Zavala et al. 2013: 344.— Humara-Gil & Cruz-Gómez 2018: 458 View Cited Treatment , fig. 4.
Type locality. Norway: Lofoten ( Calder 1991a; Schuchert 2001).
Material examined. Polyp—PB7_28, sampling site 1 (1), immature, 27°C, on algae and ascidian.
Description. Colonies stolonal. Polyps up to 2 mm high arising from creeping hydrorhiza at irregular intervals. Pedicels usually long (1082.1 μm), with 8–10 annulations at the base and 2–3 annulations below the hydrotheca, smooth in the middle. Hydrotheca cylindrical, 446.9 μm long, 232.3 μm wide at the margin, 98.4 μm wide at the diaphragm, with thin perisarc. Margin with 8–10 acute triangular cusps, slightly tilted. Hydrothecal diaphragm thin, transverse.
More detailed description in Calder (1991a), Cornelius (1995), and Schuchert (2001).
Taxonomic status. Accepted. AphiaID 117367.
Remarks. Clytia gracilis (M. Sars, 1850) has a complicated taxonomic history, resulting from the few morphological characters available for diagnosis, and wide intraspecific variation ( Cornelius 1995; Cunha et al. 2020). The polyp of the species is traditionally distinguished by its hydrotheca with pointed cusps and smooth gonotheca, in contrast with the rounded cusps and spirally grooved gonotheca usually distinctive of C. hemisphaerica (Linnaeus 1767) (see Calder 1991a; Cornelius 1995). However, recent molecular and life cycle studies have shown that some of the characters attributed to C. gracilis may be distinctive of different lineages, contributing to the validation of former synonyms and the description of new species ( Lindner et al. 2011; Zhou et al. 2013; He et al. 2015). Nonetheless, the traditional concept of C. gracilis is still known to comprise multiple cryptic lineages ( Cunha et al. 2017), and the delimitation of the typical species is still unclear. As a result, this is a tentative identification, pending more detailed studies with molecular and life cycle data.
We followed Calder (1991a) and Schuchert (2019) and considered the records of C. cylindrica from Fraser (1938a, 1948) as C. gracilis (M. Sars, 1850) . Our specimens are also very similar to specimens of C. cf. gracilis recently described by Humara-Gil & Cruz-Gómez (2018) from the coast of Oaxaca, especially regarding the size of the hydrotheca and morphology of the cusps. As noticed by Humara-Gil & Cruz-Gómez (2018), specimens of C. cf. gracilis from the Pacific coast of Mexico have smaller hydrotheca than specimens described by Calder (1991a) and Schuchert (2001, 2003). Similarly, the hydrothecal cusps of our specimens are not as tilted as the ones described by Cornelius (1995) and Schuchert (2001, 2003). Nevertheless, these characters usually present wide intraspecific variation and may not be informative for the delimitation of the different lineages of C. gracilis (see Cunha et al. 2020).
Distribution. In its traditional concept, C. gracilis is considered nearly cosmopolitan ( Cornelius 1995; Schuchert 2001), but this assumption must be revisited considering that it is a species complex (see Cunha et al. 2017). In the Pacific coast of Mexico, it has been reported in Baja California ( Isla Partida and Angel de la Guarda Islands) ( Fraser 1948), Baja California Sur (Bahía Concepción, Dewey Channel, Natividad Island and San Marcos Island ( Fraser 1938b; Fraser 1948), Sonora (Ensenada de San Francisco, Guaymas and Rocky Point) ( Fraser 1948), Nayarit (Isabel Island) ( Fraser 1938a), Jalisco (Navidad Head) ( Fraser 1938a), Guerrero (Chololo, Godornia, Morro Tigre, White Friars) ( Fraser 1938a; Salcedo-Martínez et al. 1988), and Oaxaca (Chacahua Bay and Tangola-Tangola Bay) ( Fraser 1938a; Fraser 1938c; Humara-Gil & Cruz-Gómez 2018).
Agassiz, L. (1862) Contributions to the natural history of the United States of America. Vol. IV. Little, Brown, Boston, 380 pp.
Bastida-Zavala, J. R., Garcia-Madrigal, M. del S., Rosas-Alquicira, E. F., Lopez-Perez, R. A., Benitez-Villalobos, F., Meraz-Her- nando, J. F., Torres-Huerta, A. M., Montoya-Marquez, A. & Barrientos-Lujan, N. A. (2013) Marine and coastal biodiversity of Oaxaca, Mexico. Check List, 9, 329 - 390. https: // doi. org / 10.15560 / 9.2.329
Calder, D. R. (1991 a) Shallow-Water Hydroids of Bermuda: the thecatae, Exclusive of Plumularioidea. Life Science Contributions Royal Ontario Museum, 154, 1 - 140.
Calkins, G. N. (1899) Some hydroids from Puget Sound. Proceedings of the Boston Society of Natural History, 28, 333 - 367.
Cornelius, P. F. S. (1995) North-west European thecate hydroids and their medusae. Part 2. Sertulariidae to Campanulariidae. Synopses of the British Fauna, New Series, 50, 1 - 386.
Cunha, A. F., Collins, A. G. & Marques, A. C. (2017) Phylogenetic relationships of Proboscoida Broch, 1910 (Cnidaria, Hydrozoa): Are traditional morphological diagnostic characters relevant for the delimitation of lineages at the species, genus, and family levels? Molecular Phylogenetics and Evolution, 106, 118 - 135. https: // doi. org / 10.1016 / j. ympev. 2016.09.012
Cunha, A. F., Collins, A. G. & Marques, A. C. (2020) When morphometry meets taxonomy: morphological variation and species boundaries in Proboscoida (Cnidaria: Hydrozoa). Zoological Journal of the Linnean Society, zlz 166. [published online] https: // doi. org / 10.1093 / zoolinnean / zlz 166
Fraser, C. M. (1938 a) Hydroids of the 1934 Allan Hancock Pacific Expedition. Allan Hancock Pacific Expeditions, 4, 1 - 105, pls. 1 - 15.
Fraser, C. M. (1938 b) Hydroids of the 1936 and 1937 Allan Hancock Pacific Expeditions. Allan Hancock Pacific Expeditions, 4, 107 - 127, pls. 16 - 18.
Fraser, C. M. (1938 c) Hydroids of the 1932, 1933, 1935, and 1938 Allan Hancock Pacific Expeditions. Allan Hancock Pacific Expeditions, 4, 129 - 153, pls. 19 - 21.
Fraser, C. M. (1948) Hydroids of the Allan Hancock Pacific Expeditions since March, 1938. Allan Hancock Pacific Expeditions, 4, 179 - 343, pls. 22 - 42.
He, J., Zheng, L., Zhang, W., Lin, Y. & Cao, W. (2015) Morphology and molecular analyses of a new Clytia species (Cnidaria: Hydrozoa: Campanulariidae) from the East China Sea. Journal of the Marine Biological Association of the United Kingdom, 95, 289 - 300. https: // doi. org / 10.1017 / S 0025315414000836
Humara-Gil, K. J. & Cruz-Gomez, C. (2018) New records of benthic hydroids (Cnidaria: Hydrozoa) from the coast of Oaxaca, Mexico. Zootaxa, 4455 (3), 454 - 470. https: // doi. org / 10.11646 / zootaxa. 4455.3.3
Lindner, A., Govindarajan, A. F. & Migotto, A. E. (2011) Cryptic species, life cycles, and the phylogeny of Clytia (Cnidaria: Hydrozoa: Campanulariidae). Zootaxa, 2980 (1), 23 - 36. https: // doi. org / 10.11646 / zootaxa. 2980.1.2
Salcedo-Martinez, S., Green, G., Gambo-Contreras, A. & Gomez, P. (1988) Inventario de macroalgas y macroinvertebrados benticos de areas rocosas de la region de Zihuatanejo, Guerrero, Mexico. Anales de Instituto de Ciencias del Mar y Limnologia, 15 (1), 73 - 95.
Sars, M. (1850) Beretning om en i Sommeren 1849 foretagen zoologisk Reise i Lofoten og Finmarken. Nyt Magazin for Naturvidenskaberne, 6, 121 - 211.
Schuchert, P. (2001) Hydroids of Greenland and Iceland (Cnidaria, Hydrozoa). Meddelelser om GrOnland, Bioscience, 53, 1 - 184.
Schuchert, P. (2003) Hydroids (Cnidaria, Hydrozoa) of the Danish expedition to the Kei Islands. Steenstrupia, 27, 137 - 256.
Zhou, K., Zheng, L., He, J., Lin, Y., Cao, W. & Zhang, W. (2013) Detection of a new Clytia species (Cnidaria: Hydrozoa: Campanulariidae) with DNA barcoding and life cycle analyses. Journal of the Marine Biological Association of the United Kingdom, 93, 2075 - 2088. https: // doi. org / 10.1017 / S 0025315413000969
FIGURE 7. A Plumularia floridana Nutting, 1900, nematocysts microbasic mastigophore. B–G Dynamena crisioides Lamouroux, 1824: B, colony; C, hydrocaulus; D, hydrothecae; E–F, gonothecae; G, nematocysts large microbasic eurytele. H–K Clytia cf. gracilis (M. Sars, 1850): H, polyp; I, hydrotheca; J, acute triangular cusps; K, hydrothecal diaphragm. Scale equals A—332 µm; B—0.4 mm; C, E—500 µm, D, F, H, I—200 µm; G—17 µm; J—160 µm, K—50 µm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Clytia cf. gracilis (M. Sars, 1850 )
| Mendoza-Becerril, María A., Estrada-González, Mariae C., Mazariegos-Villarreal, Alejandra, Restrepo-Avendaño, Luisa, Villar-Beltrán, Rogelio D., Agüero, José & Cunha, Amanda F. 2020 |
Clytia gracilis
| Humara-Gil, K. J. & Cruz-Gomez, C. 2018: 458 |
| Bastida-Zavala, J. R. & Garcia-Madrigal, M. & Rosas-Alquicira, E. F. & Lopez-Perez, R. A. & Benitez-Villalobos, F. & Torres-Huerta, A. M. & Montoya-Marquez, A. & Barrientos-Lujan, N. A. 2013: 344 |
Clytia attenuata
| Salcedo-Martinez, S. & Green, G. & Gambo-Contreras, A. & Gomez, P. 1988: 13 |
Gonothyraea gracilis
| Fraser, C. M. 1948: 212 |
| Fraser, C. M. 1938: 35 |
| Fraser, C. M. 1938: 109 |
| Fraser, C. M. 1938: 132 |
Campanularia attenuata
| Calkins, G. N. 1899: 350 |
Clytia cylindrica L. Agassiz 1862: 306
| Fraser, C. M. 1948: 206 |
| Fraser, C. M. 1938: 30 |
| Agassiz, L. 1862: 306 |
Laomedea gracilis M. Sars 1850: 138
| Sars, M. 1850: 138 |