Syllis ergeni, Çinar, Melih Ertan, 2005
|
publication ID |
https://doi.org/ 10.5281/zenodo.169735 |
|
DOI |
https://doi.org/10.5281/zenodo.6268754 |
|
persistent identifier |
https://treatment.plazi.org/id/177EC07A-CB2D-FF89-FEB4-A259FC963CCF |
|
treatment provided by |
Plazi |
|
scientific name |
Syllis ergeni |
| status |
sp. nov. |
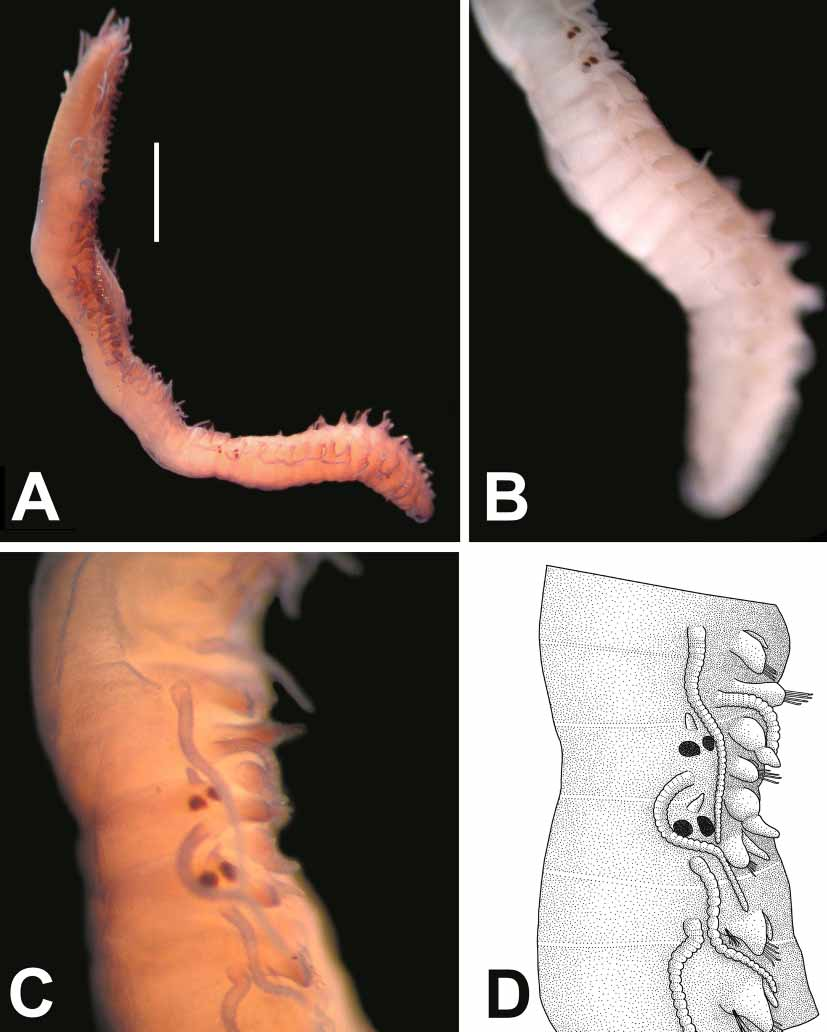
Syllis ergeni View in CoL sp. nov. ( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Material examined. Holotype: MNCN 16.01/10261, 15 January 2004, Alsancak Harbour, station 2, 0.2 m, on rocks with the mussel, Mytilus galloprovincialis , polluted environment [temperature: 13 ºC, salinity: 36.27 PSU, dissolved Oxygen concentration: 8 mg /L, pH: 8.21, NO2: 2.25 µgat/L, NH4: 9.19 µgat/L, PO4: 1.91 µgat/L]. Paratypes: ESFMPOL/04 172, 4 specimens, 15 January 2004, Alsancak Harbour, station 2, 0.2 m, among M. galloprovincialis , polluted environment [temperature: 13 ºC, salinity: 36.27 PSU, dissolved Oxygen concentration: 8 mg /L, pH: 8.21, NO2: 2.25 µgat/L, NH4: 9.19 µgat/L, PO4: 1.91 µgat/L]. Paratypes: ESFMPOL/04183, 6 specimens, 15 January 2004, Pasaport Harbour, station 3, 0.2 m, on an artificial metal platform covered with tubes of the serpulid polychaetes, Hydroides elegans (Haswell, 1883) and H. dianthus (Verrill, 1883) , and M. galloprovincialis , polluted environment [temperature: 11 ºC, salinity: 36.7 PSU, dissolved Oxygen concentration: 8.4 mg /L, pH: 8.28, NO2: 1.42 µgat/L, NH4: 2.75 µgat/L, PO4: 1.35 µgat/L]
Description. Holotype complete, without anal cirri, 6.8 mm long, 0. 44 mm wide, H+10= 1.20 mm, with 52 chaetigers. Body narrow anteriorly, enlarged in middle, gradually tapering to posterior end ( Fig. 2 View FIGURE 2 A; 3A). Ventral surface of worm flattened, dorsal surface of anterior segments domedshaped; body height as high as body width ( Fig. 3 View FIGURE 3 D). Dorsum of body between peristomium and chaetiger 20 dark brownish, then becoming pale towards posterior end ( Figs. 3 View FIGURE 3 A, B). Anterior segments (especially peristomium and chaetigers 1–5) more pigmented. Prostomium whitish, with some small brownish patches around eyes and between lateral antennae. No colour marking on dorsum of posterior segments; pale yellowish. On some paratypes, two dark brown transversal lines present on anterior and posterior parts of each anterior segment ( Fig. 3 View FIGURE 3 D). Dorsum of anterior segments with small, numerous sphaerical glands; dorsum of posterior segments with bacillary glands ( Fig. 3 View FIGURE 3 E). A few inclusions present within joints of antennae and cirri. Prostomium somewhat pentagonal, wider than long, having two pairs of reddish eyes with lenses in open trapezoidal arrangement; anterior pair (27.5 µm in diameter) larger than posterior ones (17.5 µm in diameter) ( Fig. 2 View FIGURE 2 A; 3A). No ocular specks. Median antenna longer than lateral ones, located between eyes, with 30 joints; joints indistinct, rectangular at base, rounded towards tip. Lateral antennae emerging from anterior part of prostomium, morphologically similar to median antenna, with 26 joints. Palps somewhat triangular in shape, rounded anteriorly, free at base ( Fig. 2 View FIGURE 2 A). Peristomium narrow on dorsal side, densely pigmented, with two pairs of tentacular cirri; dorsal pair with 29 joints, ventral ones with 24 joints. Dorsal cirri morphologically similar to antennae, smaller than body width, thicker and longer on anterior parapodia than those on middle and posterior ones; with ca. 32–25 joints on anterior parapodia, 34–17 joints on middle parapodia, 20–16 joints on posterior parapodia. Ventral cirri digitiform, smaller than parapodial lobe, relatively thick on anterior parapodia, thin on posterior parapodia. Parapodial lobes subrectangular on anterior segments, triangular on posterior ones. Anterior parapodia with falcigers only, numbering 12; clearly bidentate, distal tooth coarse, hook shaped, proximal tooth always conspicuous, relatively thin, slightly shorter than distal tooth; gap between distal and proximal teeth wide, crescentlike; proximal tooth with spines on cutting edge; spines on cutting edge thin, relatively thicker and longer basally, progressively diminishing in size distally; blades 30–25 µm long; shaft of falcigers with thin spines on tip ( Fig. 2 View FIGURE 2 B). Falcigers on middle parapodia numbering 9, morphologically similar to anterior ones but slightly thicker and longer; blades 32.5–25 µm long ( Fig. 2 View FIGURE 2 C). Falcigers on posterior parapodia numbering 7, with blades of 27.5–20 µm long ( Fig. 2 View FIGURE 2 D). Solitary dorsal simple chaeta, present from chaetiger 33, relatively long, thinner than shafts of falcigers, distally bifid, with short subdistal spinulation ( Fig. 2 View FIGURE 2 E). Ventral, simple chaeta present on last 6 posterior parapodia, short, curved, with subdistal spines, strongly bidentate; teeth about the same size ( Fig. 2 View FIGURE 2 F). Anterior parapodia with 3 aciculae; two aciculae morphologically similar to each other, subdistally inflated with short oblique tip, pointed distally; one aciculum thinner than others bending at nearly 90º angle at tip, rounded distally. Middle parapodia with three aciculae; two aciculae acuminate with oblique tip, pointed distally; other bending distally at 45º angle, truncated distally. Posterior parapodia with two aciculae; one acuminate, pointed distally; other bending distally at 45º angle, truncated distally. Larger specimen (paratype: ESFMPOL/04183) with 5 aciculae on anterior parapodia; four acuminate, with short oblique tip, pointed distally; one aciculum thinner than others bending distally at nearly 90º angle, with rounded tip ( Fig. 2 View FIGURE 2 G). Middle parapodia with three aciculae; two similar to each other, acuminate with oblique tip, pointed distally; other bending distally at 45º angle, with truncated tip ( Fig. 2 View FIGURE 2 H). Posterior parapodia with two aciculae; one acuminate, pointed distally; the other bending distally at 45º angle, with truncated tip ( Fig. 2 View FIGURE 2 I). Proventriculus 0.66 mm long, 0.31 mm wide, occupying 5 segments, with ca. 32 muscle cell rows. Pharynx contracted, brownish, occupying ca. 4 segments, bearing a small, triangular tooth distally; opening of pharynx surrounded by a crown of 10 soft, rounded papillae. As there is a dense pigmentation on dorsum of specimens, one paratype (4.5 mm long, 0.55 mm wide, H+10= 1.25, with 42 chaetigers) was dissected to examine its pharyngeal and proventricular structures. Proventriculus occupying 8 segments, 0.98 mm long, 0. 35 mm wide, with 38 muscle cell rows; a blackish transverse line at conjunction of each row ( Fig. 2 View FIGURE 2 J; 3F). Pharynx brownish, bearing “ Y ” shaped groove in middle, with small, triangular tooth placed just behind opening of pharynx ( Fig. 2 View FIGURE 2 J, 3C).Pygidium rounded; anal cirri missing on holotype. Paratype (6. 88 mm long, 0.65 mm wide, H+10= 1.05 mm, with 70 chaetigers) with anal cirri, with 20 distinct joints. No stylus.
Reproduction. One paratype (ESFMPOL/04172) with a stolon ( Fig. 4 View FIGURE 4 A); stock 4.75 mm long, 0.45 mm wide, H+10= 1.15 mm, with 46 chaetigers ( Fig. 4 View FIGURE 4 A, B); stolon male, 2.70 mm long, 0.55 mm wide, with 21 chaetigers. Stolon flattened ventrally, domed dorsally, whitish in colour because of sperm in coelomic cavity. Prostomium of stolon narrow with two pairs of reddish eyes, enlarged, in close trapezoidal arrangement. Anterior part of prostomium of stolon has one pair of triangular antennae and one pair of palps, which could be termed as “ tetraceros ” ( Malaquin, 1893). Palps with bulbous palpophore and long triangular palpostyle ( Fig. 4 View FIGURE 4 D). Swimming chaetae from chaetiger 2, short, smaller than parapodial lobes on chaetiger 2, slightly longer than parapodial lobes on middle and posterior parapodia, ca. 138 µm long ( Fig. 4 View FIGURE 4 D). Dorsal cirri with 33–22 joints on anterior parapodia, 20–18 joint on posterior ones. Falcigers numbering 9 on anterior parapodia, 8 on posterior cirri; bidentate; blades 20–15 µm long on anterior parapodia, 18–13 µm long on posterior cirri. Anterior parapodia with two aciculae; one acuminate, the other bending distally, with truncated tip. Posterior parapodia with one aciculum; bending distally, with truncated tip. Solitary dorsal simple chaeta from chaetiger 5, bifid, serrated subdistally. Solitary ventral simple chaeta present only on last three chaetiger, bidentate, serrated subdistally. Pygidium with two anal cirri of 21 joints, without stylus. An anomaly was observed on the paratype specimen. The last chaetiger of stock (chaetiger 47) is asymmetrical; one side bearing prostomial features of stolon (eyes, palps..etc.), the other side with a “normal” parapodial structure including chaetae, dorsal and ventral cirri ( Fig. 4 View FIGURE 4 C, D). The following segment (chaetiger 48) is the true prostomium of the stolon; each side with appendages and eyes.
Ecological features. Syllis ergeni sp.nov. was found to be associated with Mytilus galloprovincialis , Hydroides elegans and H. dianthus . Its density was 42±30 ind.m 2 (n=3) at station 2 and 50±29 ind.m 2 (n=3) at station 3. It comprised 0.71% of total polychaete individuals and 0.27% of total faunal populations at station 2, and 0.11% of total polychaete individuals and 0.08% of total faunal populations at station 3.
The biomass value (wet weight) of the specimens of Syllis ergeni ranged from 0.087± 0.06 g.m 2 (station 2) to 0.041± 0.03 g.m 2 (station 3). As hard bottom communities in the polluted harbour environment of Izmir Bay were mainly composed of clusters of Mytilus galloprovincialis and Hydroides spp., which have high biomass values, the relative importance of S. ergeni in the communities in terms of biomass value can be omitted. Hard bottom communities at the stations from where specimens of Syllis ergeni were collected were mainly dominated by the following species; Station 2: Mytilus galloprovincialis (max. density: 11750 ind.m 2), Schistomeringos rudolphi (delle Chiaje, 1828), (max. 3400 ind.m 2), Nereis falsa Quatrefages, 1865 (max. 1125 ind.m 2) and Neanthes succinea (Frey & Leuckart, 1847) (max. 800 ind.m 2); Station 3: Hydroides elegans (max. 24875 ind.m 2), S. rudolphi (max. 18325 ind.m 2), H. dianthus (max. 7975 ind.m 2), M. galloprovincialis (max. 6325 ind.m 2) and Ophiodromus pallidus (Claparède, 1864) (max. 4925 ind.m 2). All of the abovementioned species are known to be typical species of organically polluted bottoms and are widely used as pollution indicators in the Mediterranean Sea ( Ergen, 1976; Bellan, 1980).
Distribution. The type locality of this species is Izmir Bay, near Alsancak Harbour, which is one of the biggest harbours in Turkey. It holds many interoceanic cargo vessels. As Syllis ergeni has not been recorded in the area, which has been monitored since the mid 1970’s, its possible introduction to the area via ballast water and ship fouling cannot be excluded. Future reports of this species in other regions would enable us to understand its zoogeographic distribution.
Remarks. Syllis ergeni is mainly characterized by having bidentate falcigers with short blades, dark brownishreddish colour pattern on dorsum of anterior segments. Dense pigmentation on large dorsal areas of anterior segments was reported in the following Syllis species; S. barbata San Martín, 1992 , S. krohni Ehlers, 1864 , S. magdalena Wesenberg Lund, 1962, S. monilata (Imajima, 1966) , S. nigrescens Grube, 1878 , S. okadai Fauvel, 1934 ; S. schulzi (HartmannSchröder, 1960) , S. torquata Marion & Bobretzky, 1875 and S. violacea Grube, 1870 . However, all these species, except for S. krohni , S. magdalena , S. nigrescens and S. violacea , have a dense pigmentation only on a limited number of segments; only on dorsum of peristomium, chaetigers 1, 2 and 7–9 in S. okodai ; only on chaetigers 1 and 2 in S. monilata ; only on peristomium in S. schulzi ; and only on peristomium and chaetiger 1 in S. barbata and S. torquata (see San Martín, 1992; Licher, 1999). Unlike species mentioned above, S. krohni (type locality: Adriatic Sea) has dark violet pigmentation on dorsum of all anterior and sometimes middle segments. However, it differs from S. ergeni in having violet colour pigmentation (dark brownish in S. ergeni ), distally widened dorsal cirri (tapering in S. ergeni ) and unidentate blades on posterior parapodia (all bidentate in S. ergeni ). Syllis magdalena (type locality: Chile) is easily separated from S. ergeni in having unidentate falcigers. Syllis nigrescens (type locality: Phillippine) has also distinctive colour pattern (dark brownblack) ( Grube, 1878) on dorsum of anterior chaetigers, but it differs from S. ergeni in the following characters: aciculae slender, slightly acuminate in S. nigrescens vs. acuminate and benttipped in S. ergeni ; needlelike acicula present on anterior parapodia in S. nigrescens vs. absent in S. ergeni ; antennae with 20–11 joints in S. nigrescens vs. with 30–26 joints in S. ergeni ; dorsal cirri with 24–12 joints in S. nigrescens vs. with 34–16 joints in S. ergeni ; blades of falcigers measuring 20–40 µm in S. nigrescens vs. 20–32.5 µm in S. ergeni ; proximal tooth and spines of blades of posterior falcigers relatively indistinct and small in S. nigrescens vs. distinct and long in S. ergeni ; proventriculus occupying 10 segments in S. nigrescens vs. 5–8 segments in S. ergeni . The other syllid species with distinctive colour pattern is S. violacea (type locality: Red Sea) that has dark brown colour in alcohol. This species differs from S. ergeni in having dorsal cirri longer than body width (shorter than body width in S. ergeni ), somewhat slender aciculae (acuminate in S. ergeni ), long and distinctive peristomium (very short in S. ergeni ), posterior falcigers with small proximal tooth (long in S. ergeni ) and blades with a narrow gap between distal and proximal tooth (wide gap in S. ergeni ). The other species that show a close morphological affinity with S. ergeni is S. compacta , which was originally described from the Red Sea and subsequently reported from the Mediterranean Sea ( San Martín, 1984 [as Syllis golfonovoensis (Hartmann, Schröder, 1962) ], López et al., 1996; Çinar & Ergen, 2002; Çinar, 2003). However, these two species are easily separated from each other with the following characters; 1) colour (dark reddish in S. ergeni , pale brownish in S. compacta ) 2) aciculae (one acicula on anterior parapodia with tip bending at a right angle in S. ergeni , no such acicula in S. compacta ; aciculae on middle parapodia with pointed tip in S. ergeni , rounded tip in S. compacta ) 3) spines on falcigers (spines on cutting edge of falcigers small, thin, not surpassing the level of the proximal tooth in S. ergeni vs. spines on cutting edge of falcigers in S. compacta , especially those at base of blades, long, thick, surpassing the level of proximal tooth) 4) proximal tooth of falcigers (always distinct, thick and long in S. ergeni vs. small, thin and indistinct on inferior falcigers in posterior part of body in S. compacta ), 5) gap between distal and proximal tooth of blades (wide and crescent like in S. ergeni vs. very narrow in S. compacta ), 6) blade length (inferior blade lengths of falcigers of S. compacta (range: 15–19 µm) shorter than those of S. ergeni (range: 20–25 µm) 6) stylus on pygidium (present on S. compacta , absent on S. ergeni ) 7) Proventriculus (without colour pattern and occupying ca. 10 segments with more than 35 muscle cell rows in S. compacta vs. with distinctive colour pattern and occupying 5 segments with 32 muscle cell rows in S. ergeni ), 8) Dorsal cirri (wrinkled at base in S. ergeni , annulated in S. compacta ), 9) peristomium (not distinct dorsally in S. compacta vs. distinct in S. compacta ). The other species that show similarity with S. ergeni in terms of shape of aciculae and falcigers are S. remanei (Hartmann Schröder, 1960), S. warrnamboolensis (HartmannSchröder, 1987) , S. kerguelensis (Averincev, 1972) , S. kabilica BenEliahu, 1977 and S. columbretensis (Campoy, 1982) . However, S. ergeni differs from all of the abovementioned species in its distinctive colour pattern, the number of aciculae, the number of joints in dorsal cirri, the morphology and lengths of blades of falcigers and simple chaetae, and the morphology and colour of proventriculus and pharynx.
Etymology. The new species is dedicated to Prof. Dr. Zeki Ergen, from Ege University, who made excellent contributions to the understanding of polychaete diversity inhabiting the Turkish as well as eastern Mediterranean coasts.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |