Pamexis hantam Mansell & Ball
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4184.1.11 |
|
publication LSID |
lsid:zoobank.org:pub:45742D72-3E8B-4271-AD3E-0CF0C8CDF38E |
|
DOI |
https://doi.org/10.5281/zenodo.5675526 |
|
persistent identifier |
https://treatment.plazi.org/id/127187BA-F46F-FF90-FF6D-EE5B14F8FC4B |
|
treatment provided by |
Plazi |
|
scientific name |
Pamexis hantam Mansell & Ball |
| status |
sp. nov. |
Pamexis hantam Mansell & Ball View in CoL , sp. nov.
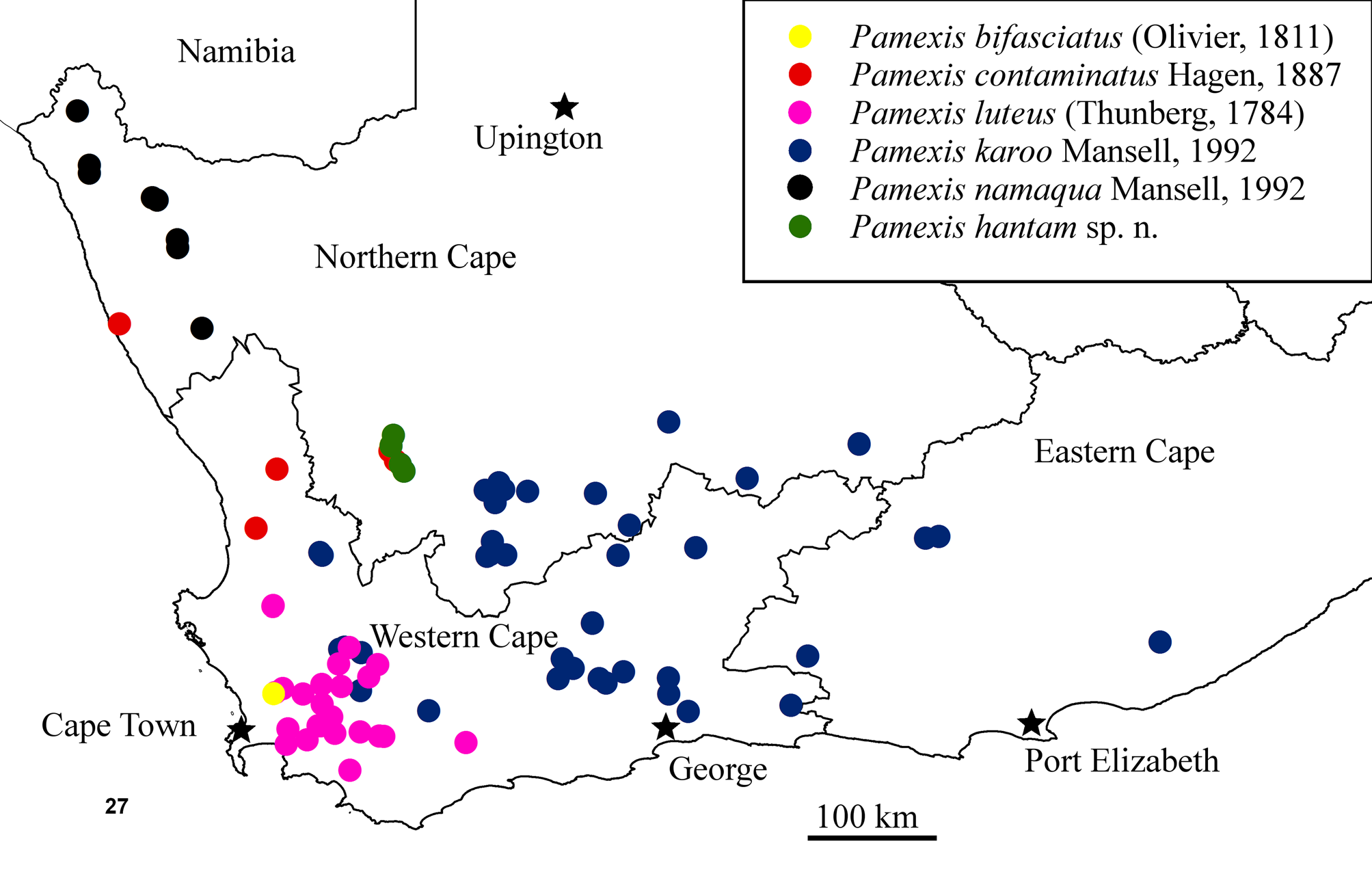
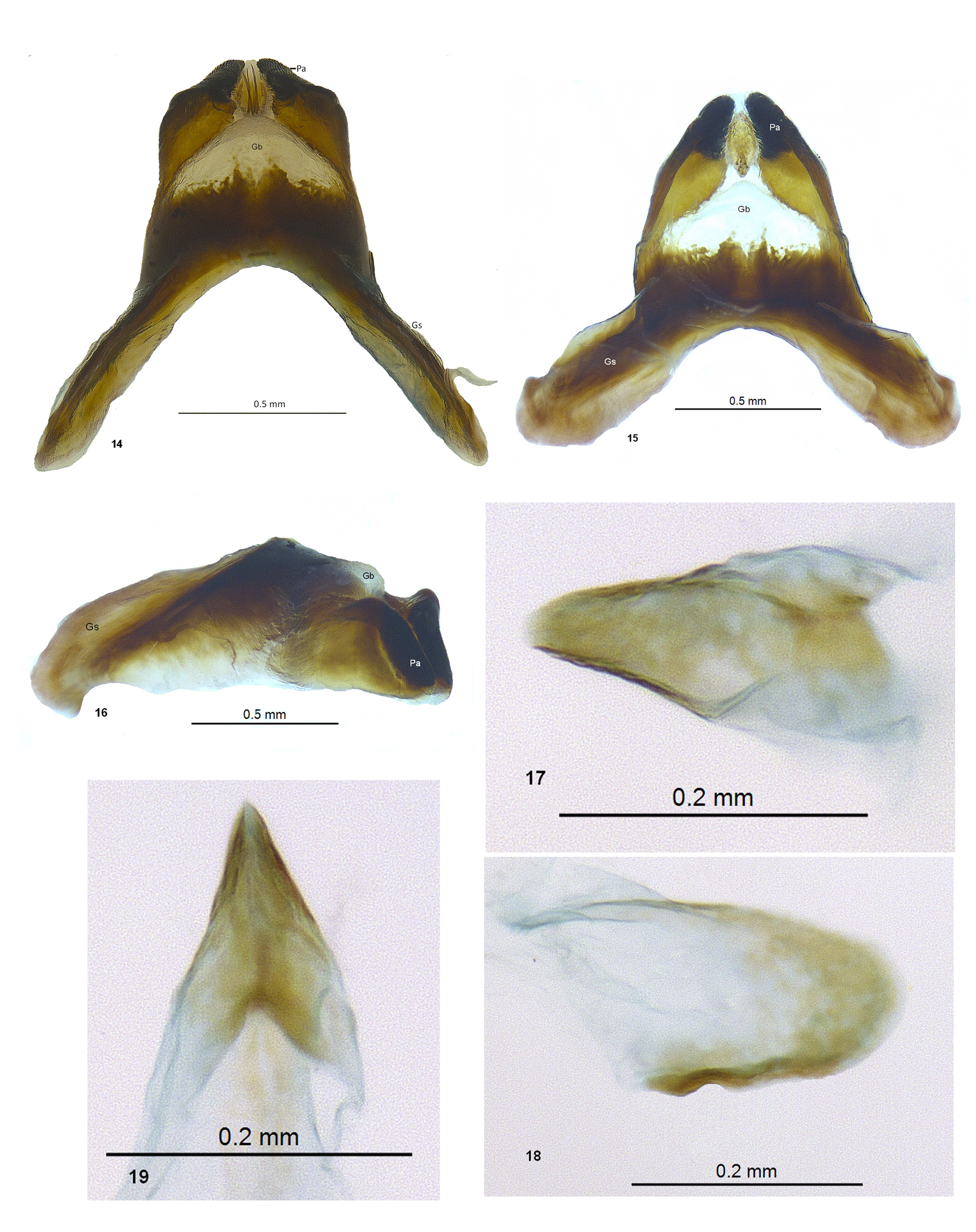
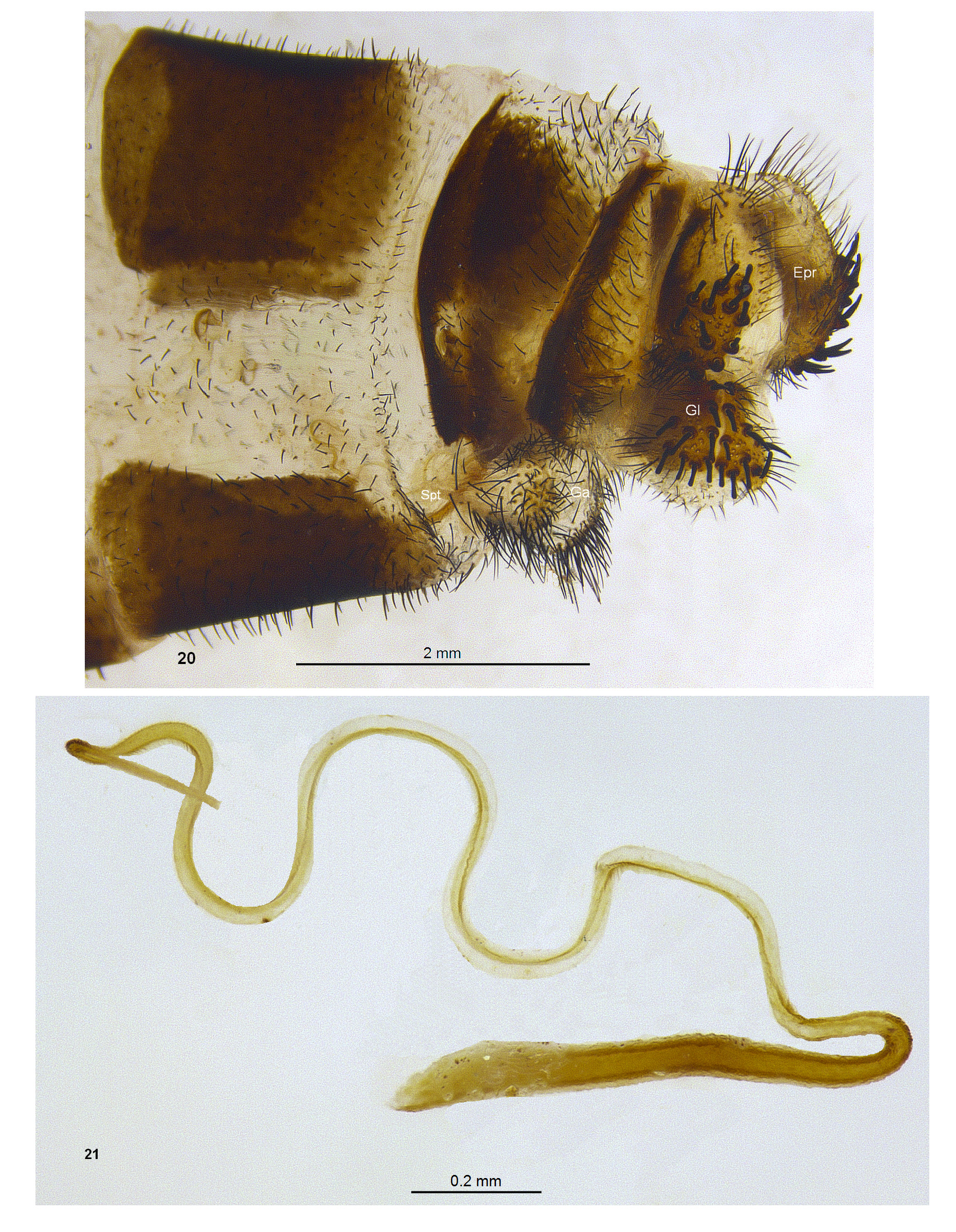
Figs 1 View FIGURES 1 – 2. 1 , 3–5 View FIGURES 3 – 5 , 9–22, 25, 26 View FIGURES 9 – 10 View FIGURES 11 – 13 View FIGURES 14 – 19 View FIGURES 20 – 21 View FIGURES 22 – 26. 22 , 27 View FIGURE 27
Etymology. Hantam , a noun in apposition, referring to the area in which the new species occurs. The name “ Hantam ” is derived from the Khoisan word!Han=ami, also previously written as ‘heyntams,’ which means “where the red bulbs grow” (TANAP 2016). This refers to a Pelargonium plant with edible roots [probably P. bifolium (Burm.f.) Willd ( Germishuizen et al. 2006)].
Description. (Based on holotype male, 12 male, 13 female paratypes)
Habitus ( Fig. 1 View FIGURES 1 – 2. 1 ). Smallish, robust, broad-winged antlions. Wings pale whitish yellow, with tessellated dark brown markings in forewings and prominent dark brown spots or bands in hind wings, but wing markings in both wings highly variable ( Figs 9–10 View FIGURES 9 – 10 ). Head, thorax and abdomen black with yellow markings on thorax. Male ectoprocts short, black, forcipate.
Head: black, slightly narrower than prothorax, vertex raised, bi-lobed with long curved black setae dorsally; antennae short, black, strongly clavate, shorter than head width, toruli slightly less than scape-width apart, torular membrane yellow, scape black with long curved setae anteriorly, flagellomeres short, uniformly black, covered with short black setae; eyes characteristically small, less than hemispherical, ocular setae absent but with long curved intermingled black and white setae antero-dorsally, short white setae ventrally and posteriorly; genae shiny black, mandibular articulations yellow, clypeus narrowly black at base, yellow distally, labrum yellowish with two black spots laterally; maxillary and labial palps short, black, terminal labial palpomere spindle-shaped with blunt apex, palpimacula round.
Thorax: prothorax almost triangular in dorsal view, with broad black central area and pale spot centrally, lateral margins broadly yellow with two black spots posteriorly, posterior margin black, long black curved setae present along anterior, lateral and posterior margins. Mesoprescutum black with yellow V-shaped mark along posterior margin; mesoscutum black with two large yellow patches anteriorly on either side of midline, two yellow triangular marks at posterior margin and a small yellow spot laterally above each wing base; long black curved setae cover mesonotum, with pale fringe of setae along posterior margin; mesoscutellum black with long white setae along posterior margin. Metaprescutum black, shiny; metascutum black, densely covered with microtrichia, two pale yellow velvety patches present on either side of midline; metascutellum shiny black with row of long white setae along posterior margin. Pleurites and sternites black, covered in long white setae, coxal articular membranes yellow.
Wings: short, broad, forewings longer than hind wings, apices rounded, hind margins smooth, membrane pale yellowish-white (greenish in living specimens, fading to pale yellow when preserved); veins pale yellowish-white with variable dark brown markings extending onto membrane ( Figs 1 View FIGURES 1 – 2. 1 , 9, 10 View FIGURES 9 – 10 ) bearing very sparse long white and black setae, hypostigmatic cells long. Forewings: with brown tessellations over most cross-veins, extending onto adjacent membrane; C with short dense black setae, some costal cells biaereolate proximally, pterostigma not discernible, Sc slightly incrassate medially with short setae along distal third, R with row of short curved black setae, slightly longer proximally; Rs arising before Cua fork, 3–7 presectoral crossveins before Rs; Mp2 (oblique vein) arising beyond Cua fork; Cup narrow not fused with A1, A2 and A3 slightly incrassate. Hind wings: pale basally with large spots sometimes coalescing into bands in distal two thirds; origin of Rs proximal to fork of Mp fork; 1 presectoral crossvein, Mp incrassate before fork; incrassate Cua arches forward at junction with posterior branch of Mp, forming the typical palparine recurrent vein; 1A incrassate.
Legs: long, black; hind legs extending to abdominal segment 4; tibiae longer than tarsi in all legs, tibial spurs robust, curved, extending to middle of T2; T1 – T4 short, T5 long, approximately equal to combined length of T1– T4; pretarsal claws longer than T1, black, slightly curved.
Abdomen: stout, slightly shorter than hind wings, completely black in male ( Fig. 25 View FIGURES 22 – 26. 22 ), covered with short black setae, in female usually black with yellow distal margins ( Fig. 26 View FIGURES 22 – 26. 22 ). Males with tergite 9 divided ( Figs 11–12 View FIGURES 11 – 13 ), sternite IX ( Fig. 13 View FIGURES 11 – 13 ) with acute but smooth apex. Ectoprocts ( Figs 11–13 View FIGURES 11 – 13 ) curved, cylindrical, with slender spines distally, long slender setae proximally, two stout spines basally; gonarcus and parameres ( Figs 14–16 View FIGURES 14 – 19 ) fused into a rigid cone-shaped structure, parameres slerotized, shiny black with medial tuft of sensory setae ( Fig. 14 View FIGURES 14 – 19 ), gonarcal bulla ( Figs 14–16 View FIGURES 14 – 19 ) translucent; hypandrium internum ( Figs 17–19 View FIGURES 14 – 19 ) lightly sclerotized, keel-shaped. Females ( Fig. 20 View FIGURES 20 – 21 ) with rounded ectoprocts bearing stout fossorial spines; lateral gonapohyses rounded with stout fossorial spines; anterior gonapophyses rounded with long slender setae; pregenitalae ( Fig. 22 View FIGURES 22 – 26. 22 ) triangular, sclerotized; spermathecae ( Fig. 21 View FIGURES 20 – 21 ) slender, tapering distally.
The small sclerotized triangular pregenitale of the female, which is situated in membranous folds between the anterior gonapophyses serves to accommodate and guide the equally inconspicuous keel-shaped hypandrium internum of the male to the spermatheca during sperm transfer.
Larvae: unknown.
Distribution. Northern Cape Province, South Africa.
Systematic position. Pamexis hantam is morphologically similar to P. namaqua ( Fig. 2 View FIGURES 1 – 2. 1 ) and they are clearly adelphotaxa. Mansell (1992) stated that P. namaqua was most closely related to P. contaminatus Hagen , but this relationship has now been superseded by discovery of P. hantam , which is clearly closer to P. namaqua than to P. contaminatus . Pamexis hantam is distinguished from P. namaqua by its larger size and wing length and by the markings on the thorax and black abdomen ( Figs 25, 26 View FIGURES 22 – 26. 22 ). The wings of P. hantam are longer and less rounded than those of P. namaqua , and also lack the diffuse brown suffusion manifest in P. namaqua ( Fig. 2 View FIGURES 1 – 2. 1 ). The males are easily separated by the black ectoprocts of P. hantam compared to the yellow ectoprocts of P. namaqua ( Fig. 23 View FIGURES 22 – 26. 22 ) and the extensive yellow markings on the abdomen of the latter ( Figs 23, 24 View FIGURES 22 – 26. 22 ). There appears to be no overlap in the currently known distributions of the two taxa and the adults also have disparate phenologies.
Habitat and behaviour. This species occurs over a recorded range of about 25 km from the summit of the Hantam Mountain (Hantamberg) (over 1500 m) southwards to Keiskie Pass and beyond (at 1220 m). The vegetation type on the Hantam Mountain is Hantam Plateau Dolerite Renosterveld (FRd2), while the vegetation type near Keiskie Pass is Roggeveld Shale Renosterveld (FRs3) ( Mucina & Rutherford 2006). Both are in the Fynbos Biome. This Pamexis species occurs at the highest altitude (1200–1500 m) of the genus, where the frost incidence is 10–40 days per year. Summers are very hot, with a mean daily maximum temperature of 31.1° for February ( Mucina & Rutherford 2006).
The unifying feature of all the known localities is the presence of white and black crustose lichens on the rocky landscape with low shrubland and rich herblands containing an abundance of geophytes ( Mucina & Rutherford 2006). When settled on the crustose lichens on rocks, these alert insects are exceptionally difficult to detect. The thallus of the lichens is a light whitish/green colour, with the discs of the cup-shaped, lecideine apothecia, being black. These colours are consequently very similar to those of P. hantam , with the rounded dark coloration of the fruiting bodies (apothecia) also being very similar in size and shape to many of the ovoid black markings on the fore- and hind-wings ( Figs 3–5 View FIGURES 3 – 5 ). The crypsis/camouflage is enhanced by the extensive pale setae on the head and thorax, which minimize an edge shadow from the visible portion of the body when resting on the lichen covered rocks during the day (see Fig. 4 View FIGURES 3 – 5 ). When disturbed, these rapid-flying diurnal antlions take to the air and are carried by the prevailing winds, settling up to 70 meters away. They usually settle on rocks or the ground. They occasionally also settle on vegetation. Many of the low shrubs in the area of occurrence have grey/green leaves, including Eriocephalus sp. (‘kapokbossie’) and the ‘renosterbos’ ( Elytropappus rhinocerotis ), again enhancing camouflage. In flight they strongly resemble the local and very widespread butterfly Belenois aurota aurota , the brown-veined white (family Pieridae ). The long flight episodes possibly lessen the chance of asilid attack. The rapid transition from a settled and camouflaged insect on a rock to a white flash-pattern on flight is probably an effective anti-predation ‘startling’ attribute.
No oviposition sites nor early stages of any of the taxa in this genus are currently known.
Material examined. Holotype ♂, NEUR 10999 , SOUTH AFRICA, Northern Cape Province, Keiskieberg Pass (31.35.26S 19.51.52E, 1176m), 18.xii.2010, M.W. Mansell & J.B. Ball ( SANC).
Paratypes: 1♀, same data as holotype ( SANC) ; 2♂ 1♀, NEUR 10706 , same locality but 16.xii.2009, J.B. Ball ( JBBC & SANC) ; 1♂ 1♀, NEUR09689, Hantamberg (31.20.03S 19.48.07E, 1533m), 16.xii.2004, J. White (JBBC); 1♂ 2♀, JBNE 00541 , same data but 20.xii.2006 ( JBBC) ; 4♂ 5♀, NEUR 09761 , same data but 21.xii.2006 ( JBBC) ; 1♂ 2♀, NEUR 11001 , same locality but 19.xii.2010, M.W. Mansell & J.B. Ball ( SANC) ; 1♂, NEUR11000, Keiskie Farm, 31.39.12S 19.53.51E, 1254m, 18.xii.2010, M.W. Mansell & J.B. Ball (SANC); 2♂ 1♀, NEUR 11075 , same data but 10.i.2011, A.P. Marais ( SANC) .
Additional material: 1♀ (Photograph), Akkerendam Nature Reserve, Calvinia (31.25.38S 19.46.57E), 18.xii.2014, C.K. Willis (VM279)*; 1♀ (Photograph), same data and photographer but 23.xii.2015 (VM9363).
* VM refers to the Virtual Museum Number, Animal Demography Unit, University of Cape Town: http:// vmus.adu.org.za/?vm=LacewingMAP-279 and 9363.
| SANC |
Agricultural Research Council-Plant Protection Research Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |