Icospina, Watson & Carvajal & Sergeeva & Pleijel & Rouse, 2016, Watson & Carvajal & Sergeeva & Pleijel & Rouse, 2016
|
publication ID |
https://doi.org/ 10.1111/zoj.12390 |
|
persistent identifier |
https://treatment.plazi.org/id/0D7BAC7F-8720-264C-9B0D-FC026CAFF9D7 |
|
treatment provided by |
Marcus |
|
scientific name |
Icospina |
| status |
gen. nov. |
M ICOSPINA AURIBOHNORUM GEN. ET SP. NOV.
FIGS 5A–E View Figure 5 , 6A–E View Figure 6 , 7F, G; TABLE View Figure 7 2 VIGTORNIELLA SP. AGUADO ET AL. (2013: 614)
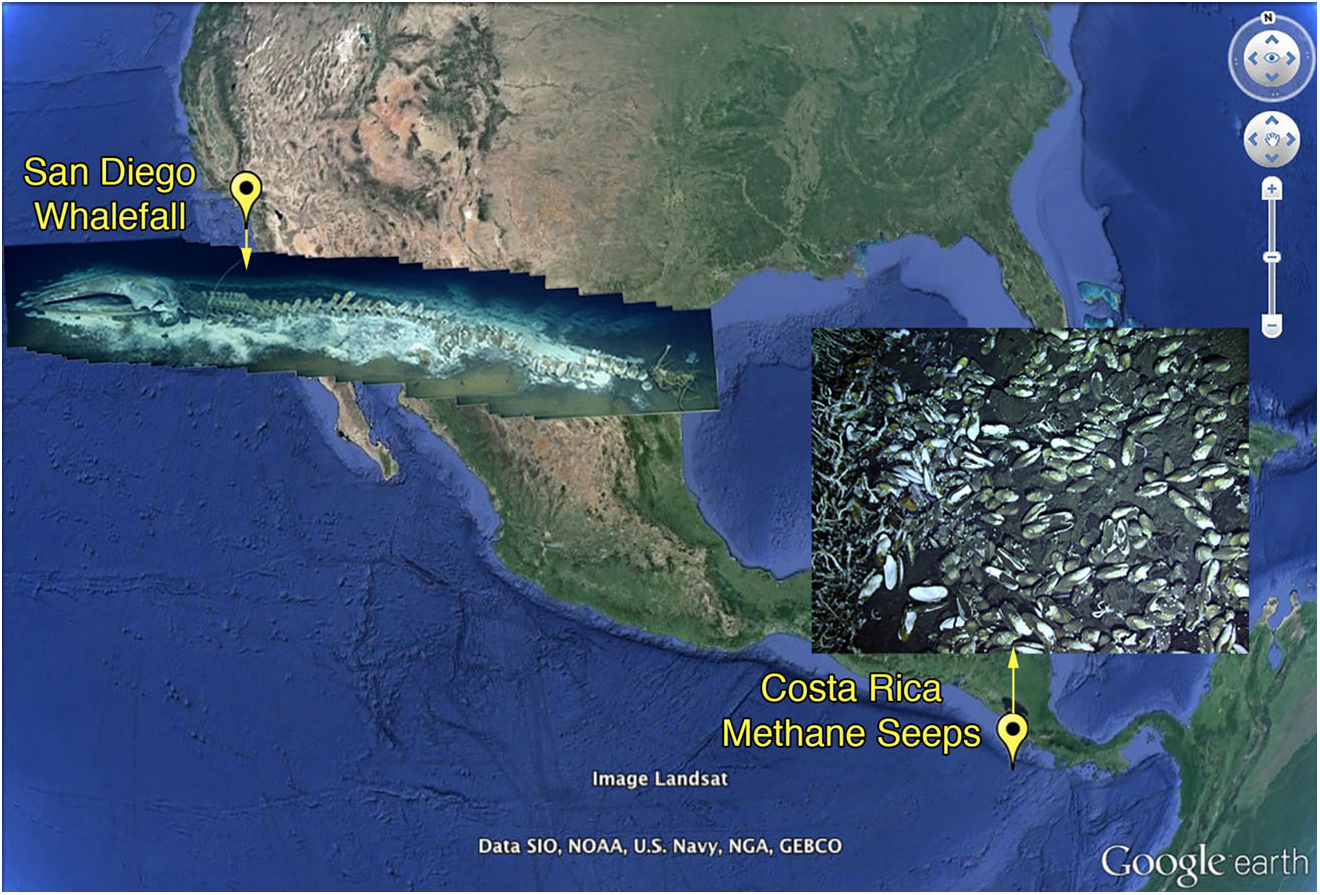
Material examined. Type material: Holotype, eastern Pacific Ocean , 12 miles west of Mission Bay , San Diego, 32 ° 46 ′ 37.08 ″ N, 117 ° 29 ′ 15.24 ″ W, whale fall (fin whale), 842 m, collected ROV Doc Ricketts, dive 471, 18 May 2013, 8NE (was 24E before cutting for DNA sequencing), anterior end ovigerous female, length when complete 3.7 mm, width 2 mm, SIO-BIC A3641 ; paratype, 1; 8NE (was 15E before cutting for DNA sequencing), length when complete 1.6 mm, width 1.1 mm, SIO-BIC A3640 . GoogleMaps
Additional material: Costa Rican Margin, methane seeps, HOV Alvin, dive 4589, 08 ° 55.790 ′ N, 84 ° 18.723 ′ W, Mound 12, 998 m, two females 1; 16E, length 1.8 mm, width 1 mm; 1; 12NE, anterior end, length 1.35 mm; width 0.95 mm, ethanol, SIO-BIC A1934 ; 1, formalin, SIO-BIC A1935 ; AD 4510, 09 ° 10.335 ′ N, 84 ° 47.920 ′ W GoogleMaps , Jaco Summit, 745 m, 3 Apr 2009, DNA, SIO-BIC A1598 ; two, osmium, SEM photos, SIO-BIC A1599 ; two, formalin, SIO-BIC A1427 ; HOV Alvin, dive 4509, 09 ° 07.030 ′ N, 84 ° 50.550 ′ W GoogleMaps , Jaco Scarp, 1866 m, active carbonate rock (S2) with bacterial mat, 3 Apr 2009, CR534, 3NE, SIO-BIC A5853 ; HOV Alvin, dive 4504, 11 08 ° 55.250 ′ N, 84 ° 18.323 ′ W GoogleMaps , Mound 11, 1040 m, active rock bacterial mat, RC 388AD, 25 Feb 2009; HOV Alvin, dive 4511, 12 08°55.832 ′ N, 84°18.738 ′ W,
Mound 12, 998 m, inactive rock, rock S2, 5 Mar 2009. Note: individuals from the last two sites were destroyed ( Levin et al., 2015).
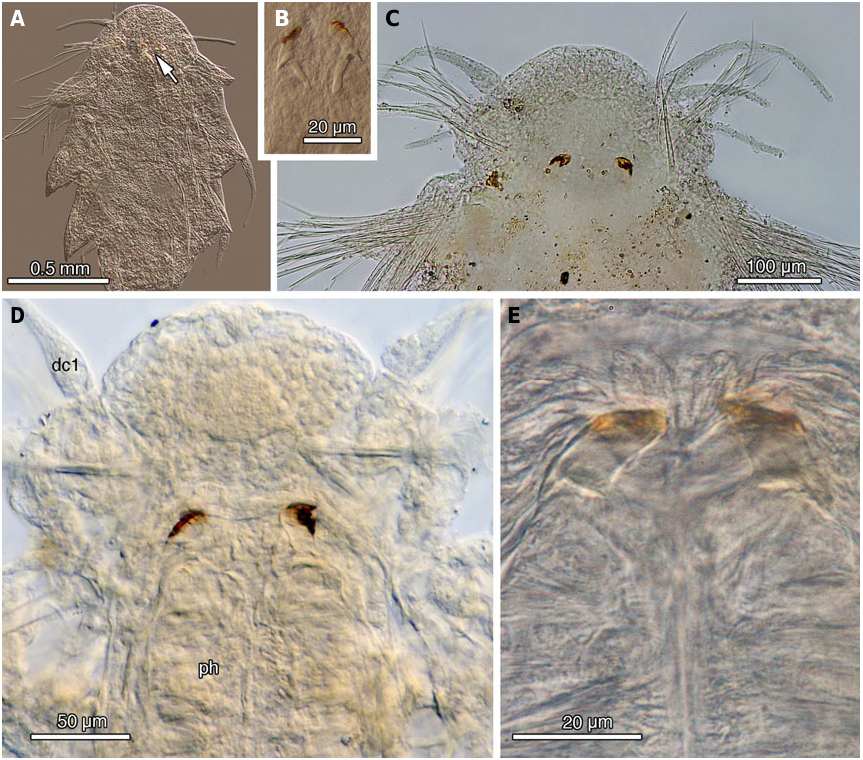
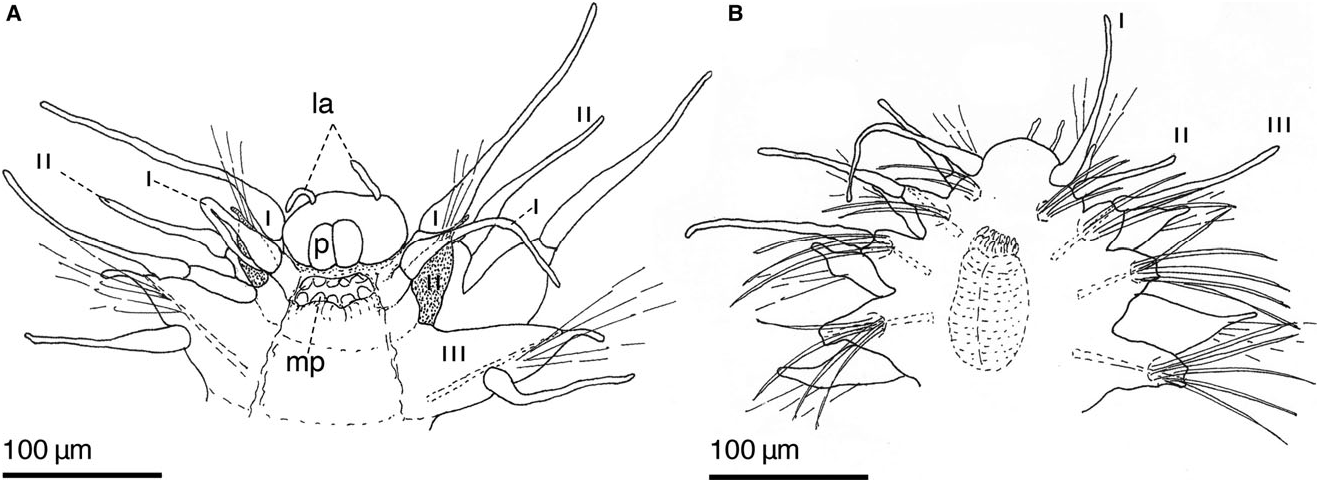
Description. Body shape elongate, slightly tapered posterior and anterior ends. Epidermis covered with tiny rounded bacteria; ciliary patches on prostomium (nuchal organs?) and segmentally ( Fig. 6A View Figure 6 ). Live specimens pale yellow with anterior five segments more reddish; long fascicles of golden notochaetae; longer fascicles of golden neurochaetae directed laterally. Parapodia deeply incised; dorsum bare ( Fig. 5A–E View Figure 5 ). Prostomium rounded with two digitiform lateral antennae inserted anteroventrally; palps very small, spherical, inserted ventrally directly anterior to mouth. Antennal length little longer than palps. Proboscis with distal papillae; no discernible jaws present. Segment I with two pairs of long fusiform dorsal cirri and slightly shorter ventral cirri; aciculae and chaetae absent. Segment II notopodia with small notochaetal fascicle, shorter dorsal cirri compared with the following cirri; very small neuropodia directed anteriorly with small neurochaetal fascicle of slender compound spinigers, ventral cirri absent ( Figs 6C View Figure 6 , 7F, G View Figure 7 ). Notopodia midbody with long, slender dorsal cirri; notochaetae composed of longer and shorter slender spines, with very fine, double row of serrations, more visible on distal third, curved tip ( Fig. 6B View Figure 6 ). Dorsal, ventral aciculae long and stout. Neuropodia with pronounced prechaetal lobe, long ventral cirri. Neurochaetal compound falcigers with long shafts; bifid joint. Superior two or three falcigers with long, slender blades with slight curved distal tip; mid to lower slender blades slightly shorter with serration to tip ( Fig. 6D, E View Figure 6 ). Oocytes of various sizes, all less than 80 µm in diameter.
Remarks. Anaerobic oxidation of methane by microbial activity facilitates the precipitation of authigenic carbonate at methane seeps off Costa Rica. Fauna living at both ‘active’ and ‘inactive’ carbonate sites depend on chemosynthetic-based nutrition ( Levin et al., 2015). Chrysopetalids were present in moderate numbers at both types of carbonate site in Costa Rican seeps, and comprised two species, M. auribohnorum gen. et sp. nov. (this study) and Chrysopetalinae gen. et sp. nov. (C. Watson & G. Rouse, pers. observ.). Micospina auribohnorum gen. et sp. nov. lacks jaws and is found amongst high-density organismal assemblages with an isotope reading of Carbon –35.6, Nitrogen 6.6, a signal close to that of animals feeding on sulphide-oxidizing bacteria ( Levin et al., 2015).
Micospina auribohnorum View in CoL gen. et sp. nov. exhibited no discernible morphological differences in collections made over a depth range of ~ 750–1800 m at Costa Rican methane seeps. Mature individuals from Costa Rican seeps ( Fig. 5C–E View Figure 5 ) are slightly smaller than the mature female holotype found from the whale carcass off San Diego ( Fig. 5A View Figure 5 ). However, morphological data and nearly identical COI sequences (holotype, SIO-BIC A3641, GB KU057938 View Materials ; paratype, SIO-BIC A3640, GB KU05- 7937; Costa Rica specimen, SIO-BIC A1427, GB JX0- 93564) indicate that they are the same species ( Figs 2, 5 View Figure 5 – compare 5A, with 5C–E; Table 2), even though there is ~ 5500 km between the Costa Rican seep and the San Diego whale fall sites ( Fig. 1 View Figure 1 ). Presumably, stepping-stone habitats exist between San Diego and the Costa Rican sulphide-based communities, either as whale falls or methane seeps. The egg size seen in M. auribohnorum View in CoL gen. et sp. nov. was maximally around 80 µm ( Fig. 5A, D View Figure 5 ). Although it is not known if these were mature, they are much larger than the maximum observed size found in V. zaikai View in CoL (40 µm; Kiseleva, 1992; N. Sergeeva, pers. observ.), which is inferred to have lecithotrophic development Murina (1997). Further data on egg sizes and development are clearly needed as the reported egg sizes are unlikely to support extended lecithotrophic development. Nevertheless, if V. zaikai View in CoL is any indication ( Fig. 3A View Figure 3 ), then M. auribohnorum View in CoL gen. et sp. nov. may have the potential for a long larval life.
Previous deep-sea polychaete molecular studies have shown no apparent subdivision between widely separated north-east Pacific deep-sea populations, north and south of the equator. Examples include the amphinomid Archinome levinae Borda, Kudenov, Chevaldonne, Desbruyeres, Blake, Fabri, Hourdez, Shank, Wilson, Pleijel, Schulze and Rouse, 2013 View in CoL , recorded from sedimented vents in Guaymas Basin and methane seeps at the Costa Rican Margin ( Borda et al., 2013), and the ampharetid Amphisamytha fauchaldi Solıs-Weiss & Hernandez-Alcantara, 1994 View in CoL , which occurs at these sites as well, but also extends much further northwards to Oregon methane seeps ( Stiller et al., 2013). Other polychaetes that were thought to be widely distributed have been shown to be cryptic species, however, such as the polynoid Bathykurila guaymasensis Pettibone, 1989 View in CoL initially described from sedimented vents in Guaymas Basin, and then later from whale falls off California in the Santa Cruz Basin ( Glover et al., 2005), although specimens from the type locality have yet to be sequenced.
Habitat and distribution. Micospina auribohnorum gen. et sp. nov. has an eastern Pacific distribution along the continental margin at methane seeps off the Costa Rican Margin and from whale bones off San Diego, with a depth range of 745–1866 m.
Etymology. The species name auribohnorum is a conjunction between the Latin aurum for ‘gold’, referring to the distinctive notochaetal colour, and for the Bohn family, for their support of the Scripps Benthic Invertebrate Collection and whale fall research.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
Icospina
| Watson, Charlotte, Carvajal, J. Ignacio, Sergeeva, Nelly G., Pleijel, Fredrik & Rouse, Greg W. 2016 |
Micospina auribohnorum
| Watson & Carvajal & Sergeeva & Pleijel & Rouse 2016 |
M. auribohnorum
| Watson & Carvajal & Sergeeva & Pleijel & Rouse 2016 |
V. zaikai
| Watson & Carvajal & Sergeeva & Pleijel & Rouse 2016 |
V. zaikai
| Watson & Carvajal & Sergeeva & Pleijel & Rouse 2016 |
M. auribohnorum
| Watson & Carvajal & Sergeeva & Pleijel & Rouse 2016 |
Archinome levinae
| Borda, Kudenov, Chevaldonne, Desbruyeres, Blake, Fabri, Hourdez, Shank, Wilson, Pleijel, Schulze and Rouse 2013 |
Amphisamytha fauchaldi Solıs-Weiss & Hernandez-Alcantara, 1994
| Solis-Weiss & Hernandez-Alcantara 1994 |
Bathykurila guaymasensis
| Pettibone 1989 |