Desmophyllum dianthus ( Esper, 1794 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1018.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:02411A3D-C81F-4E3D-A24F-8D07FF9A64C4 |
|
persistent identifier |
https://treatment.plazi.org/id/0D5E87EC-A303-0A1D-9376-03D0C37C092C |
|
treatment provided by |
Felipe |
|
scientific name |
Desmophyllum dianthus ( Esper, 1794 ) |
| status |
|
Desmophyllum dianthus ( Esper, 1794) View in CoL
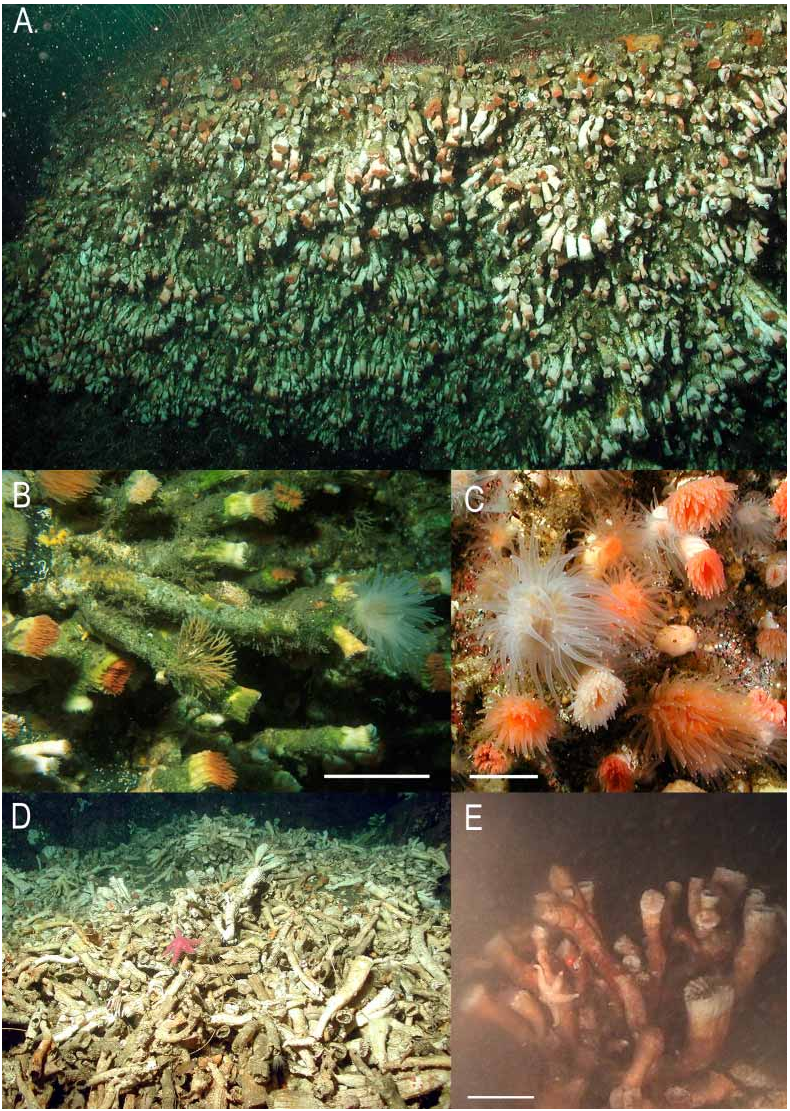
Figs. 5A–E View FIGURE 5 , 6F–H View FIGURE 6 , 7 View FIGURE 7
Madrepora dianthus Esper, 1794 : pl. 69.
Desmophyllum cristagalli Milne Edwards & Haime, 1848: 253 View in CoL , pl. 7, figs. 10, 10a.. — Squires, 1969: 17, pl. 6, map 1. — Cairns, 1982: 29–30, pl. 8, figs. 9–12, pl. 9, figs. 1–3, map 6. — Stanley & Cairns, 1988: 236, figs. 2, 3A.
Desmophyllum cumingii Milne Edwards & Haime, 1848: 254 View in CoL , pl. 7, fig. 11.
Desmophyllum ingens Moseley, 1881: 160–162 View in CoL , pl. 4, figs. 1–6, pl. 5, figs. 1–4a. — Squires, 1969: 17, pl. 6, map 1.
Desmophyllum dianthus View in CoL . — Cairns, 1995: 26–27, pl. 9a–d (description, synonymy). — Piñón, 1999: 20, 81. — Försterra & Häussermann, 2001: 155; 2003: 119–128, colour figs. 2–5 (20 sites in south Chilean fjords, 8–45 m). — Försterra et al., 2005: 937–977, colour figs. 2–5.
New Records. —Caleta Gonzalo (42°32'46.6"S, 72°37'00.2"W), Chile, 28 m, 23 Feb 2001, 1 specimen, USNM 1009656 About USNM ; Caleta Gonzalo , Chile , 35 m, 7 Feb 2001, 1 specimen, USNM 1009657 About USNM ; Lenca (41°38'20.4"S, 72°40'07.4"W) GoogleMaps , 27 m, 1 specimen, USNM 1009654 About USNM ; ship wreck close to Puyuhuapi , Chile , 24 m, 13 Feb 2001, 1 corallum, ZSM; Isla
Cailin, Chile, 8 m, 3 Feb 2001, 1 specimen, ZSM; Juan Fernandez Islands , Sector El Pangal, Bahia Cumberland, 4–5 m, March 1998, 1 specimen, IZUACNI0070; Mouth Seno Baker (close to Tortel 47°49'S, 73°34'S since the Rio Baker is flowing in there), Chile, 300 m, MZUC8133; Moyano Coll. 1101972; Isla Inocentes, 150 m (50°33'S, 74°53'W); MZUC8136; Moyano Coll. 9101972; Isla Diego Ramírez, South Chile, 100 km S of Cape Horn, 1900 m, on antipatharian, Sept 2001, 1 specimen, IZUACNI0019; Caleta Gonzalo, Reñihue Fjord (42°2'46,6''S, 72°37'0,2''W), Chile, 25–35 m, 23 Feb 1997, 2 pseudocolonies, ZSM 20020240 View Materials ; Caleta Gonzalo, Reñihue Fjord (42°2'46,6''S, 72°37'0,2''W), Chile, 25–35 m, 23 Feb 2001, 2 pseudocolonies, RMNH Coel. 32192; Punta Llonco, Comau Fjord (42°20'28''S, 72°26'54''W), Chile, 25–30 m, 6 Aug 2003, 2 pseudocolonies, IZUACNI0039 and 0040 GoogleMaps .
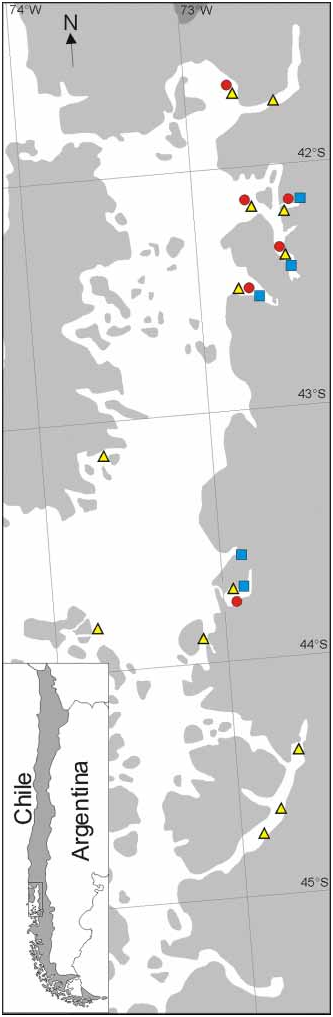
Distribution. —Chilean fjord region: Seno de Reloncaví: Punta Chaica/Lenca (S1); Fiordo Comau: numerous localities including Lilihuapi Island at the mouth (S2); Fiordo Quintupeu (S3); Fiordo Reñihue: several localities (S4); Isla Cailín/Quellón (S5); Fjord Pitipalena (S6); Bahía Santo Domingo (S7); Seno Ventisquero (S8); Canal Puhuhuapi (S9), Estero de Reloncaví (S11), Guaitecas Islands (S12), Mouth Seno Baker, Isla Inocentes, Isla Diego Ramirez; Juan Fernández Islands; 8–1900 m.
Remarks. — Moseley (1881) was the first to report D. dianthus (as D. ingens ) from four localities off Chile between 48°S – 53°S latitude at depths of 256– 631 m. The only other reports of Chilean D. dianthus (as D. cristagalli ) were those of Cairns (1982), consisting of 7 additional records from latitude 46°S – 54°S and depths of 91–821 m, as well as from a seamount on the Chile Rise (Eltanin 326), and specimens reported by Försterra and Häussermann (2003) and Försterra et al. (2005) from the Chilean fjord region (8–45 m), further documented herein. Cairns (1982) and later Stanley & Cairns (1988) suggested that quasicolonial D. ingens was so abundant at certain stations that it probably served as the foundation species for deepsea coral banks. The specimens reported herein extend the known distribution to 41°S at the remarkably shallow depth of 8 m. D. dianthus is a cosmopolitan species, the previous depth range known for it being 25–2460 m, the shallow range from New Zealand fjords ( Cairns 1995). The shallowwater Chilean specimens are of the “ ingens ” form of the species, consisting of quasicolonial coralla up to a record length of 40 cm ( Försterra & Häussermann 2003), and having a thin theca and widelyspaced dissepiments, which results in a very low density corallum. The larger specimens have a sixth cycle of septa arranged: S1–3>S4>S5>S6.
The extreme morphological differences between specimens of Desmophyllum dianthus , cosmopolitan distribution and the extremely different habitats and ecological niches that this species inhabits make it imaginable that we are dealing with several species or subspecies that show a morphological continuum. Molecular studies might help to resolve this question (Fukami et. al. 2004).
Polyp morphology. —In general only the most apical portion of the corallite is covered with polyp tissue. Tissue color of lips and tentacles can vary from almost clear transparent
over white to bright orangered; tissue of the oral disc is generally clear transparent; pinkish, yellow or greenish appearance results from endolithic algae. Pharynx in some individuals with longitudinal orange stripes. Spherulae only slightly pronounced, whitish.
Growth forms and rates. —Specimens in shallow water show variability in growth form. Solitary specimens, specimens in loose aggregations, and coralla at the border of dense aggregations show short, massive and trumpetshaped corallites with ratios of PD:GCD of generally less than 1:2. In these specimens, at least the primary septa are prominent and project beyond the border of the cup. Specimens within dense aggregations are generally pseudoramified, with elongated, delicate, cylindrical corallites of up to 400 mm long, with the CD rarely exceeding the diameter of the pedicel. In these specimens, the septa do not or hardly exceed the calice border. The latter growth form is probably caused by intraspecific competition for a position of the polyp exposed to current. Closely neighbored corals often partially fuse and elongated specimens often exhibit “adventive roots” which reinforce the frequently slender base. On vertical and nearvertical walls, and at sites with higher sedimentation, specimens of D. dianthus show a downward bending growth. In Chilean fjords below 20 m, specimens of D. dianthus may form dense aggregations that have been observed to cover more than 1000 m 2. Especially under overhangs these aggregations can obtain a three dimensional structure through extensive growth of a single individual and pseudobranching, which can include up to five generations. Here population densities may exceed 1500 individual per m 2. Specimens on the fibre hull of a boat wreck in 22 m depth, Seno Ventisquero (S 8), allowed for minimum growth rate estimations: the largest specimens measured 21 mm in height and 15 mm in diameter. Taking into account the time of the boat accident, the maximum age of these specimens must have been less than 9 years with resulting maximum growth rates of 2.3 mm/yr length growth and approx. 1.6 mm/yr diameter growth. These growth rates of young individuals are considerably higher than those estimated for deep Atlantic specimens ( Risk et al. 2002).
Habitat. —During our diving surveys we found this species on primary (rock walls, boulder ground, fibre ship hull) and biogenic hard substratum (mussel shells) where the slope exceeds approx. 80° with moderate to strong currents exposure during tide changes. We regularly found this species in the euphotic zone between 20 and 45 m depths; single specimens can be found as shallow as 8 m (S5, S2), but always below the regular influence of the low salinity layer. The largest and most shallow accumulations of these corals were found in the fjords, particularly in the fjords Comau (including fjord Quintupeu and Lilihuapi Island) and Reñihue (S2–4). Habitat temperatures ranged from 8 to 13.5 °C and salinities from 28.5 to 34 ‰. ROV observations showed similar structured, partly very large coral banks down to 250 m. At these depths some of the corallites showed a brown cover which probably consists of iron/manganese compounds which indicates anoxic conditions.
Associated species. —We regularly found specimens with corallites stained by endolithic algae which give them a pinkish, yellowbrown, olive or greenish appearance. The endolithic algae seem to be restricted to the portion of the corallite that is still covered with polyp tissue. This might indicate a more than just a commensal relationship ( Försterra et al. 2005). Pinkish staining is restricted to the more light exposed side of the corallite. Desmophyllum dianthus was regularly associated with calcified and noncalcified crustose red algae (probably genus Lithothamnium or Lithophyllum) which can cover the substratum around the corallites, but were never observed to cover the corallite itself. Further common species that share the habitat with D. dianthus are the serpulid polychaete Apomatus sp. , the sponges Geodia magellani , Mycale thielei and Iophon sp. , the bryozoan Cellaria malvinensis , the gastropod Crepidula sp. and the coral Caryophyllia huinayensis which may grow between as well as on specimens of D. dianthus . In depths greater than 100 m D. dianthus is associated with giant clams of the genus Acesta . The proximal portion of the corallite that is not covered by polyp tissue, in many specimens is densely covered with epizoic organisms of various taxonomic groups. Close to coral banks we often observed large schools of the scorpaenid fish Sebastes capensis with especially the younger specimens hiding between the corals when disturbed. In exposed, not overhanging portions which are not suitable for D. dianthus , the sea anemone Actinostola chilensis is regularly found in vicinity to coral banks ( Häussermann, 2005). An important number of specimens show perforations in the proximal portion, mainly caused by sabellid polychaetes and boring sponges of the genus Cliona .
Specimens from Juan Fernandez Island were collected in 4–5 m depths in small rocky caves.
Mortality and coral rubble. — Epizoic load, especially through other corallites of the same species, and elongated fragile growth in combination with erosive work of perforating organisms can cause older corallites to brake off. On vertical walls, further corals can break off when hit by a falling pseudocolony causing a domino effect. As a consequence, accumulations of coral rubble can be found on terraces below coral banks, with the uppermost individuals generally still being alive.
In the shallow water of the fjords Desmophyllum dianthus is mainly nocturnal.
In the fjords, this species can generally be found on overhanging portions of the substrate where it generally dominates the benthos below 20 m. Only at sites with low or moderate sedimentation and moderate to strong tidal currents less dense accumulations can be found on near vertical to vertical substrate, where they generally show downward bending growth. These facts imply high sensitivity towards sedimentation stress. An increment of sediment stress caused by salmon and mussel farms might represent a serious threat to these unique shallow water coral communities ( Försterra & Häussermann 2003). In this context the association with the fish species Sebastes capensis might represent a mutualistic symbiosis. The fish has been observed to use the spaces between corallites in denser coral accumulations as safe hiding places whereas the corals might benefit from being cleaned from covering sediment through the sweeping effect of the fish movements.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Desmophyllum dianthus ( Esper, 1794 )
| Cairns, Stephen D., Häussermann, Verena & Försterra, Günter 2005 |
Desmophyllum dianthus
| Forsterra, G. & Beuck, L. & Haussermann, V. & Freiwald, A. 2005: 937 |
| Forsterra, G. & Haussermann, V. 2003: 119 |
| Forsterra, G. & Haussermann, V. 2001: 155 |
| Pinon, G. C. 1999: 20 |
| Cairns, S. D. 1995: 26 |
Desmophyllum ingens
| Squires, D. F. 1969: 17 |
| Moseley, H. N. 1881: 162 |
Desmophyllum cristagalli
| Stanley, G. D. & Cairns, S. D. 1988: 236 |
| Cairns, S. D. 1982: 29 |
| Squires, D. F. 1969: 17 |
| Milne Edwards, H. & Haime, J. 1848: 253 |
Desmophyllum cumingii
| Milne Edwards, H. & Haime, J. 1848: 254 |