Assa wollumbin, Mahony & Hines & Mahony & Moses & Catalano & Myers & Donnellan, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.5057.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:41511A38-335B-4A12-BF5F-4A5FED1A86D5 |
|
DOI |
https://doi.org/10.5281/zenodo.5705532 |
|
persistent identifier |
https://treatment.plazi.org/id/03FE7139-FF8E-FFBF-A8A1-FC3C13C6551C |
|
treatment provided by |
Plazi |
|
scientific name |
Assa wollumbin |
| status |
sp. nov. |
Assa wollumbin sp. nov.
Mount Wollumbin hip-pocket frog
Figs 12 View FIGURE 12 , 13 View FIGURE 13 .
Holotype: AMS R185959 adult male, collected on the forest floor at Wollumbin (Mt Warning) National Park, 28 o 23’ 55” S, 153 o 17’ 01” E, north-eastern New South Wales, by Stephen Mahony , on 17 October 2014. GoogleMaps
Diagnosis. Assigned to Assa based on the generic definition of Tyler (1972).
Assa wollumbin sp. nov. can be distinguished by apomorphic nucleotide states at 30 sites in the ND2 gene ( Tables 2 View TABLE 2 , 3 View TABLE 3 ).
Measurements of holotype (mm). SVL 15.8; HL 4.7; HW 6.3; ED 1.9: EN 1.1; IND 2.2; FL 10.2, TL 6.6; HHL 4.3; FLL 2.6.
Description of holotype ( Fig. 12 View FIGURE 12 ). AMS R185959. An adult male. Small (SVL 15.8 mm), body pear-shaped, flattened dorso-ventrally, with a prominent dorso-lateral margin demarcated by a strong contrast in colouration and the presence of a line of small tubercles that extends from behind the eye to the posterior third of the dorsum. The pouch opening is on the dorso-lateral line in the anterior groin but is usually hidden by the thigh when the animal is at rest ( Fig. 14E–F View FIGURE 14 ). Brood pouch openings on both upper lateral posterior flanks.
Head moderately wide (HW/HL = 1.4). Canthus rostralis slightly concave; nostrils closer to tip of snout than to eyes; internarial distance greater than distance from external naris to eye (IND/EN = 1.8); snout slightly pointed when viewed from above; downward wedge shaped when viewed laterally. Eyes moderately long (ED/HL = 0.4), prominent, not protruding in dorsal and ventral views; pupil horizontal and elliptical. Tympanum present but obscured by granular skin, and substantially smaller than the eye.
Glandular ridge of enlarged tubercles from rear of jaw to axil. Skin of dorsal and lateral surfaces including limbs, finely granular. Ventral surfaces including limbs coarsely granular covered with small tubercles and lateral edges and margin of jaw with more prominent tubercles. Urostyle prominent and rounded and upturned above the cloaca. Posterior, hidden surface of the thighs with many moderately sized rounded tubercles and strongly contrasting in colour with the dorsal and ventral surfaces.
Arms moderately long, thin, with a row of moderate sized tubercles on the posterior dorso-ventral margin of the forearm. Fingers short without webbing; finger relative lengths 3> 2> 4> 1; finger one greatly reduced, only one phalanx; tips of all fingers slightly expanded laterally and smooth and rounded below; palmar surfaces with a large round and flattened tubercle at the outer heel; subarticular tubercles on each phalanx of the fingers.
Legs moderately long (TL/SV = 0.4), thigh robust and muscular. Row of prominent tubercles on the posterior dorso-lateral margin of the tarsus and heel. Foot relatively long (FL/SVL = 0.65). Toes unwebbed, relative lengths 4> 3> 5> 2> 1; tips of all toes simple slightly expanded laterally and smooth and rounded below; rounded subarticular tubercles at base of each phalanx; small rounded outer metatarsal tubercle.
Variation. A small frog, females are slightly larger than males (SVL: males range 13.1–19.3 mm, females range 14.7–20.5 mm) ( Table 5 View TABLE 5 ). Head moderately wide to wide (HW/HL: range 1.2–1.7). Eye size variable (ED/HL: range 0.3–0.5); internarial distance substantially greater than distance from eye to naris (IND/EN: range 1.6–2.5). Hind limbs moderately long (TL/SVL: range 0.4–0.5).
Distinct dorso-lateral ridge formed by rounded tubercles that vary in size between individuals from small to prominent. Some individuals with one or two larger rounded tubercles coincident with the v-shaped markings on the dorsum. Tubercles along the posterior dorso-lateral margins of the tibia and tarsus, and larger tubercles at the elbow and heel, more prominent in some than others.
Colour and pattern. Highly variable dorsal and ventral colouration and patterning, no obvious differences between the sexes ( Figs 12 View FIGURE 12 , 13 View FIGURE 13 ). Base colour of the dorsal surface of head, body, legs and arms highly variable from light cream with an orange tinge to reddish brown to a darker brown and brown-black ( Figs 12 View FIGURE 12 , 13 View FIGURE 13 ). In many individuals with a lighter colouration there are distinct darker brown to black markings, which in many individuals occur as two anterior direct v-shaped darker chevrons on the upper and lower dorsum. The lower marking is continuous with a transverse dark bar on the thigh and foot when folded next to the body ( Figs 12 View FIGURE 12 , 13 View FIGURE 13 ). In other individuals there is a broad darker mid-dorsal stripe with distinct broad lighter cream and black margins ( Fig. 13F View FIGURE 13 ). The mid-dorsal stripe may be mottled. A faint interorbital bar that does not extend up onto the orbit in most individuals, and in those individuals with a mid-dorsal stripe it forms the anterior margin.
The face may be the same colour as flanks to a darker hue and may be immaculate or with ill-defined black mottling. Canthus rostralis with or without a darker marking between the anterior margins of the eye to the nostril. A dark vertical bar beneath the eye to the maxilla in most specimens, may be obscured in darker individuals, and occasionally with a cream lined edge. In most individuals there is a distinct delineation between the dorsal surface and the flanks marked by a ridge that is accentuated in individuals where the colouration of the dorsum and flanks is highly contrasting. Often there is a thin lighter coloured line on the ventral side of the ridge. Colouration of the upper lateral surface is dark brown to black diffusing into a lighter brown towards the ventral surface.
Ventral surfaces including groin, hind and forelimbs, hands and feet, posterior surface of thigh and chin are the same colour within an individual ( Fig. 13B, G–I View FIGURE 13 ). However, the colour differs considerably among individuals, from darker brown-black with small lighter coloured granules that are more prominent on the edges of the mandibles, to light burnt-yellow with darker flecks. Iris black with a fine golden inner rim and fine golden flecks dorsally.
Reproductive mode and tadpole description. The occurrence of the hip-pockets in the male is a distinctive form of sexual dimorphism, and the opening of the pouch can be identified readily in the field with the use of a hand lens. The lateral pouch opening in the inguinal region is up to 3 mm long, runs laterally and is inclined posteriorly at a slight angle on the upper dorso-lateral margin. The pouch is continuous with the dermis and extends forward into the lymph sac beneath the lateral and ventral surfaces. The right and left pouches are independent and do not join. There is no evidence of a nuptial pad on the dorsal surface of the fingers or prepollex in any of the male specimens that were collected while calling and reproductively active.
The mating sequence has been observed on more than ten occasions during a study of male parental care in Assa conducted at Wollumbin (MJM pers. obs.). When calling, males expand the skin below the chin, however the vocal sac is not loose (see Fig. 14A View FIGURE 14 ). Males call from secluded positions usually beneath dense leaf litter, from curled-up leaves and in crevices among small rocks ( Fig. 14B View FIGURE 14 ). Males establish a calling position and females are attracted to the male who does not move away from the calling position.After a female has been attracted to the calling position, the male rapidly moves to an inguinal embrace. After embrace the female moves away, with male attached, to the site of oviposition which is up to 20 cm from where the male called. This indicates that the male is not calling from a microhabitat that represents a preferred oviposition site. Oviposition occurs beneath leaf litter or in a similar hidden location that is moist but not in standing water. The small clutch of large eggs is placed closely, the eggs adhere to one another and there is usually at least two layers ( Fig. 14C, D View FIGURE 14 ). Embryonic development proceeds for a period of at least seven days, and the developing embryo can be seen through the thick transparent jelly coat ( Fig. 14D View FIGURE 14 ). The adult male takes up a position about two or three body lengths away from the clutch, also under the leaf litter and in a position where he can view the clutch ( Fig. 14E View FIGURE 14 ). At times the male will move to the clutch and move over it. We can only speculate on the cues that the males rely on to indicate the time of embryo hatching. However, the embryos do wriggle vigorously at times and this may provide some cue for the male. At hatching the male moves over the clutch, and the jelly liquifies. The embryos wriggle vigorously, with the head arched downward. The male rotates slowly within the clutch, and the legs are held slightly away from the body so that a crevice is created between his flanks and legs. The flanks are slightly concave. Newly hatched tadpoles wriggle up the crevice to the opening of the pouch in the inguinal region ( Fig. 14F View FIGURE 14 ). Apart from rotating in the liquified clutch the male does not assist tadpoles into the pouch. Our observations are that most carrying males (n=12) have approximately equal number of tadpoles in the left and right pouch. Males have a rounded and robust body habitus when carrying tadpoles, and the pouch opening is no more distinct than in non-carrying males. Metamorphosis occurs in the pouches and the young emerge, usually hind limbs first ( Fig. 14F View FIGURE 14 ), without any prompting or assistance, such as body contractions by the male.
Our observations of embryonic and tadpole development (n = 6) in A. wollumbin sp. nov. are very similar to those described in detail for A. darlingtoni across its range by Anstis (2013). However, we observed two aspects of reproductive behaviour not described previously. First, we found several cases in which a male carried two cohorts of tadpoles, and the difference in development stage was such that it can only be that the male had incorporated two clutches that were fertilised well separated in time. In these cases, the brooding male was actively calling when collected. Second, when a female approached a male to enter into amplexus we did not see any evidence that the female inspected the male prior to embrace as described by Ehmann & Swan (1985). The two observations are linked in that we observed no evidence that the female inspects the male to see if he is holding tadpoles in his pouches prior to mating, and as such this is direct evidence that a male carrying young will mate a second time in the one breeding season.
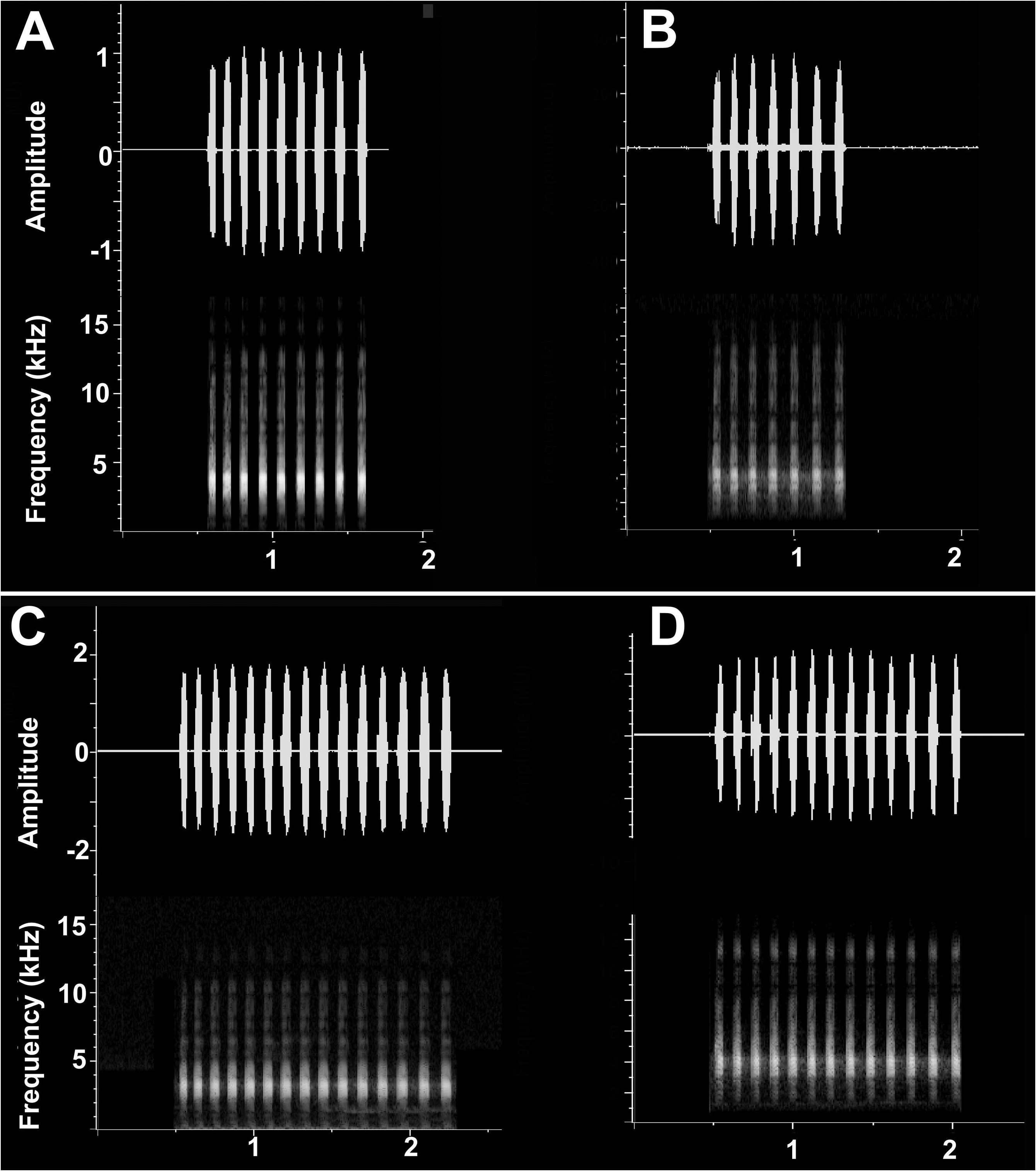
Call. Male advertisement calls have a duration of about 1.2 s (mean 1.24 s; range 0.8– 1.8 s) and comprise a series of fully modulated repeated notes of the same duration (mean 0.046 s; range 0.045–0.05) separated by an inter-note interval that is slightly longer (mean 0.046 s; range 0.045–0.05) ( Fig. 6 View FIGURE 6 ). Inter-note time is even across most of the call but is slightly longer prior to the last note. The number of notes ranges from 5 to 14 ( Table 6 View TABLE 6 ). There is only very slight amplitude modulation in the call with the first few notes being lower in energy than the subsequent notes. The call is broadband with a relatively high dominant frequency (mean 3743, range 3593–3992 Hz), and the lower mean 5% and upper mean 95% frequencies (3093 and 4218 Hz respectively), with the lowest and highest frequencies from 2906 to 14514 Hz ( Fig. 6 View FIGURE 6 ). There is no evidence of frequency modulation across the call or within notes ( Fig. 6 View FIGURE 6 ).
Temporal and structural attributes of the call are affected by temperature. Call duration, inter-call duration and NRR are significantly negatively correlated with temperature ( Table 6 View TABLE 6 ), while the number of notes in the call is positively correlated with temperature (Supplementary Table S2 View TABLE 2 ). Structural and temporal attributes of the call can also be affected by behavioural context. Males synchronise calls in Assa darlingtoni and can add or delete notes from the call and alter the timing between calls and adjust the note repetition rate ( Clulow et al. 2017).). We have observed synchronous calling in A. wollumbin sp. nov.
The courtship call is made when a male is approached closely by a female, at amplexus and possibly at times when the males are in very close proximity (< 5 cm) (MM pers. obs.). This call is often produced independently, however it may also precede, by a short time interval (< 0.2 s) the advertisement call. This call is of short duration (mean 0.59 s ± SD 0.43) and produced at the same dominant frequency (mean 3640 Hz) as the advertisement call ( Fig. 7 View FIGURE 7 ). Individual pulses cannot be distinguished, and the note is amplitude modulated with a rapid rise to a plateau prior to rapid decay, without evidence of frequency modulation.
Etymology. Referring to the distribution, which is primarily on Wollumbin, a noun in apposition. Wollumbin is the name given by the First Nation Traditional Custodians for the Tweed, and the mountain that is the cone of the Tweed volcano (also known as Mount Warning).
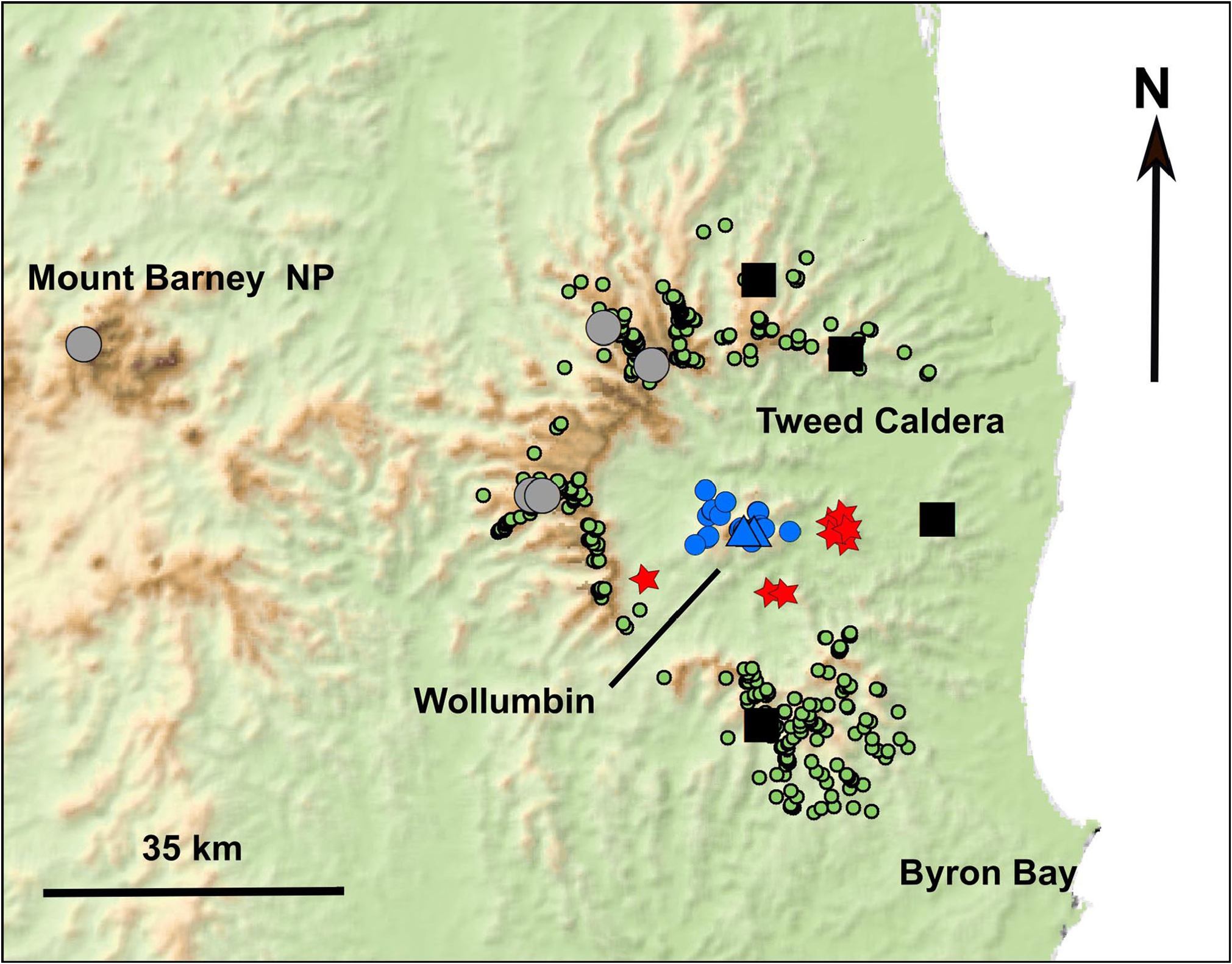
Distribution. Known only from Wollumbin (Mt Warning) in north-eastern NSW ( Fig. 8 View FIGURE 8 ). The discovery of two species of Assa , in close proximity in the Tweed Volcano region, with one on the central volcanic cone Wollumbin and the other on the surrounding caldera and its slopes, is unexpected. The distance between sampling locations on Wollumbin and those on the caldera on four side are between 12 and 17 km ( Fig. 8 View FIGURE 8 ). The caldera is about 32 km in diameter, and Wollumbin is in the centre, however there is significant relief within this small distance with Wollumbin ( 1068 m above sea level [asl]) and the higher parts of the caldera ( 1040 m asl), are separated by the caldera valley below 100 m asl.
The natural barrier to gene flow between A. wollumbin sp. nov. on Wollumbin and A. darlingtoni on the caldera to the east, south, west and north are unknown. A preference for cool temperate forest at higher altitudes and avoidance of lower and hotter forests does not appear to be the likely barrier. While Assa are commonly found in the cooler temperate forests at higher altitude, they also occur at lower altitudes in subtropical rainforest in the Currumbin Valley ( 160 m asl), at Terania Creek, Nightcap Range ( 220 m asl) and on Wollumbin ( 420 m asl). Prior to European settlement and clearing of parts of the Tweed Valley, the natural vegetation was continuous between Wollumbin and the higher altitudes of the caldera rim ( McDonald 2010, McDonald & Hunter 2010). Indeed, there is an almost continuous closed forest connection across this barrier in several locations at the present time, and this is especially the case along the small streams where the riparian zones have an almost complete rainforest understorey. Regardless of the removal of forest connections in the past 200 years, the level of genetic differentiation uncovered means that the break in gene flow between A. wollumbin sp. nov. and A. darlingtoni greatly pre-dates these events.
It is possible that A. wollumbin sp. nov. and A. darlingtoni occur sympatrically in locations that have not been sampled and that a reproductive isolating mechanism prevents gene flow, or that the current geographic barriers are not what drove the speciation. We found no evidence of sympatry in the genetic screening between the species, however the extent of our sampling on Wollumbin and its surrounding foothills is limited ( Fig. 8 View FIGURE 8 ). We are uncertain about the species identity of Assa from three locations (Mount Nullum and Wollumbin State Forest) within the Tweed Caldera within close proximity of Wollumbin ( Fig. 8 View FIGURE 8 ), and identification of samples from these locations would provide a firmer understanding of the distribution of the two species within the caldera.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.