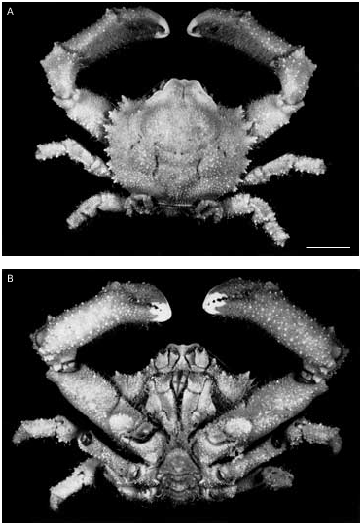
Cryptodromiopsis plumosa ( Lewinsohn, 1984 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.4689208 |
|
DOI |
https://doi.org/10.5281/zenodo.4885329 |
|
persistent identifier |
https://treatment.plazi.org/id/03FE211D-FFC8-EF46-FD71-E651FE3E39AD |
|
treatment provided by |
Felipe |
|
scientific name |
Cryptodromiopsis plumosa ( Lewinsohn, 1984 ) |
| status |
|
Cryptodromiopsis plumosa ( Lewinsohn, 1984)
? Dromidiopsis plumosa Lewinsohn, 1984: 104 View in CoL , fig. 3a-g. Dromidia plumosa – McLay 1991: 470.
Cryptodromiopsis plumosa – McLay 1993: 190, fig. 17f. MATERIAL EXAMINED. — Guam. Apra Harbour, Glass Breakwater, 13°27’N, 144°47’E, 3-6 m, among rocks, 8.XI.1993, coll. H. T. Conley, 1 7.2 × 6.8 mm (sponge cap) (GUM 304) (ZRC2000.2112).
SIZE. — Maximum cw for males is 13.3 × 11.7 mm. The specimen from Guam is one of the first females to
Dynomenids and dromiids ( Crustacea, Decapoda, Brachyura ) from the Pacific
be collected. Another larger female from Hawaii is reported by McLay (2001). Maximum female size is 10.5 × 9.5 mm. C. plumosa carries sponge caps.
DEPTH AND HABITAT. — Previously known depth range was 16- 55 m. The specimen from Guam, 3-6 m, extends the range to much shallower depths.
DISTRIBUTION. — Recorded from the Seychelle Islands, Chesterfield Islands, New Caledonia and now Guam. C. plumosa has also been reported from Hawaii (see McLay 2001). This is clearly a widespread, shallow water, Indo-Pacific species.
DISCUSSION
In his original description of this species Lewinsohn (1984) was uncertain about the genus in which it should be placed. The type specimen is a male and Lewinsohn believed that he needed a female specimen to be sure that it belonged in the genus Dromidiopsis . Hence the use of? Dromidiopsis . But he had made an error in stating that there was an epipod on the cheliped, so that the new species should in fact have been placed in the genus Cryptodromiopsis . A distinctive feature of C. plumosa is its shaggy appearance, the result of a dense pile of long plumose setae. Also the propodal spines of the legs are distinctive. There is a small distal spine on the inferior margin of the propodi of p2 and p3. On p4 and p5, which are used to carry pieces of camouflage, the spines are numerous and well-developed. The dactyl of p4 is opposed by two or three spines, with one or two spines on the outer propodal margin. On the p5 the dactyl is opposed by two spines with three spines on the outer propodal margin and an accessory spine on the dactyl itself. The presence of large numbers of spines on the p4 and p5 is regarded as being close to the ancestral condition (see McLay 1993). Description of the female characters is given in McLay (2001).
Cryptodromiopsis unidentata ( Rüppell, 1830) View in CoL ( Fig. 8 View FIG )
Dromia unidentata Rüppell, 1830: 16 View in CoL , pl. 4, fig. 2, 2a, pl. 5 fig. 9. — Lewinsohn 1984: 107.
Cryptodromiopsis unidentata View in CoL – McLay 1993: 192, figs 7a-k, 18a; 1998: 347.
MATERIAL EXAMINED. — Guam. Agana Bay, 13°27’N, 144°47’E, reef front, north of boat basin channel, on dead consolidated coral, 12 m, 30. V.1986, coll. R. K. Kropp, 1 3.6 × 3.7 mm (unidentified compound ascidian cap) ( GUM 229). — Piti Lagoon, among silty dead coral, 2 m, 22. V.1993, coll. H. T. Conley, 1 8.3 × 7.9 mm (unidentified sponge cap) ( GUM 262). — Piti Reef, 0.5-2 m, among rocks, 4-18.VIII.1993, coll. H. T. Conley, 1 7.7 × 7.5 mm (sponge cap, Petrosia sp.), 2 5.9 × 6.0 mm (sponge cap belonging to the Desmacellidae , genus could not be determined), 9.4 × 8.9 mm ( GUM 298). — Piti Lagoon, 1 m, bomb holes in reef flat, VI.1995, coll. J. Starmer, 1 14.2 × 14.0 mm (soft coral cap, Sinularia sp.) ( UGI no registration number). — Apra Harbour, 20-25 m, American Tanker, 8.IV.1997, coll. T. Leberer, 1 10.4 × 10.5 mm (sponge cap, Iotrochota purpurea (Bowerbank, 1875)) ( UGI 6020).
Tonga. Ha’api Group, Lifuka Island, 19°50’S, 174°22’W, 6-10 m, 7.XI.1996, coll. G. Paulay, 1 6.9 × 6.8 mm (soft coral cap, Xenia sp.) ( UGI: BTON-4).
SIZE. — The maximum cw for male C. unidentata is 34.0 mm and for females it is 31.0 mm. All the specimens reported here are much smaller than the maximum size.
DEPTH AND HABITAT. — The known depth range for C. unidentata is 0-100 m, with most specimens collected from less than 50 m. All the present specimens came from within this range.
DISTRIBUTION. — C. unidentata is a widespread Indo- West Pacific species known from the Philippines and as far south as the Kermadec Islands, north of New Zealand, but it has not previously been recorded from Tonga.
DISCUSSION
Cryptodromiopsis unidentata has been described and illustrated by McLay (1993) where a full synonymy can be found. The distinctive features of C. unidentata are: a carapace about as wide as long, the lack of any teeth on the anterolateral carapace margin and the dense mat of fine setae on the carapace where they form a “fringe” across the frontal region.
A feature of the camouflage behaviour of C. unidentata is the diversity of organisms used to cover itself with. The most common organisms are sponges and ascidians (both solitary and compound), but soft corals and actinians have also been recorded. Often the crab is deeply embedded in its camouflage so that only the pereopods are visible. The specimens collected from Guam and Tonga were carrying pieces of the sponges Iotrochota purpurea (Bowerbank, 1875) , Petrosia sp. and a desmacellid, two soft corals, Sinularia sp., and Xenia sp. and an unidentified compound ascidian. A notable feature of the present specimens is the size of the pieces of camouflage carried by the crabs. For the crab carrying the soft coral, Xenia sp., the cover ratio was 2.81 and for the crab carrying the other soft coral, Sinularia sp. the ratio was 2.35. The height of these coral caps was 10 mm and 50 mm respectively. The crab carrying the compound ascidian had a cover ratio of 3.9 but the cap was only 4.0 mm high. The two crabs carrying Petrosia sp. and the desmacellid sponge had cover ratios of 3.9 and 4.6 and the height of these caps was 5.8 mm and 3.5 mm respectively. The crab carrying the large piece of dark purple sponge, Iotrochota purpurea , was dwarfed by its camouflage and had a very strange appearance. The cover ratio was 20.6 and the sponge was around 15 mm high. In this case the crab was not holding the sponge in the middle, but had hollowed out a cavity almost at one end, so that most of the sponge hung over one side in a very asymmetrical way. It was the bizarre appearance of this crab that drew the attention of the divers who collected it. Unless the sponge had some offensive properties it is difficult to conclude that the crab was well-protected beneath its cap.
The importance of the ratio of cap area: crab size lies in the fact that it is an indication of the amount of room available for increase in the size of the crab when it next moults. A cover ratio of less than 1.0 indicates that the crab is not well-covered, a ratio of just 1.0 is perhaps the minimum for camouflage, while anything above 1.0 provides room for crab growth. Thus the crabs carrying the soft corals and the sponges, Petrosia sp. and a desmacellid, had ratios of 2.35 to 4.6, which probably gave adequate cover. The crab carrying the large piece of Iotrochota purpurea had a cover more than four times as large as its body. The use of sponges and ascidians by Cryptodromia hilgendorfi has been investigated by McLay (1983).
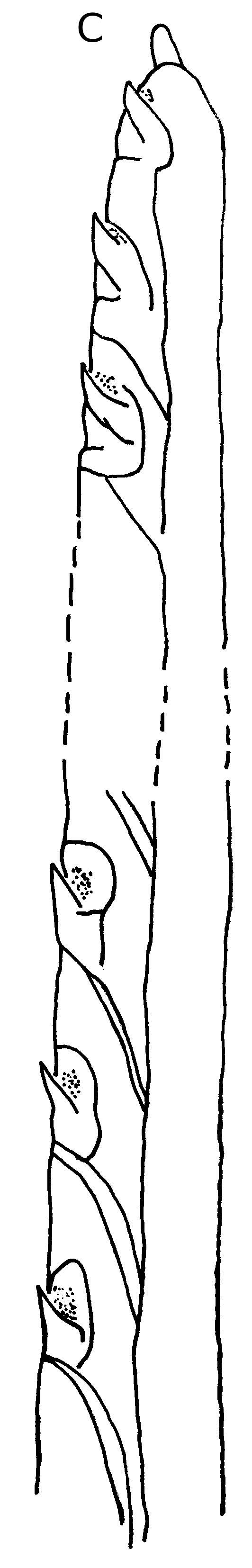
Genus Takedromia McLay, 1993 DIAGNOSIS. — Carapace distinctly wider than long, surface moderately to strongly convex, granulated or tuberculated, may be areolated. Rostrum tridentate, projecting, may be truncated, lateral teeth usually thin and eave-like. Anterolateral teeth well-developed, lacinated or tuberculated, posterolateral borders dentate or tuberculated. Coxae of third maxillipeds separated by a wide gap and inserted well forward of tip of sternum on a triangular plate. Female sternal grooves end apart between bases of first legs. Antennal exopod well-developed, prominent median distal spine on second segment, all antennal segments minutely denticulated. Cheliped without an epipod, male chelipeds much larger than those of female. First two pairs of legs tuberculated and granulated, inner margins of dactyls armed with up to five small spines. Last two pairs of legs very small, third pair shortest, dactyli of both pairs opposed by single propodal spines, none on outer propodal margin. Abdomen of six free segments. Uropod plates well-developed, visible externally, used in abdominal locking mechanism by fitting in front of serrated flanges on base of first legs. Male telson rounded or sub-truncate. Abdominal segments adorned with granules and or tubercles. First male gonopod semi-rolled tube, sharply tipped and setose, second gonopod simple, needle-like (after McLay 1993).
Takedromia cristatipes ( Sakai, 1969) ( Fig. 9 View FIG )
Cryptodromia cristatipes Sakai, 1969: 245 View in CoL , pl. 1, fig. 1; 1976: 18, text fig. 10.
Takedromia cristatipes View in CoL – McLay 1993: 212, figs 9a-b, 19a-b.
MATERIAL EXAMINED. — Guam. 13°27’N, 144°47’E, no site details, 13.III.1975, 1 22.5 × 18.5 mm ( UGI no registration number, ZRC 2000.0755).
Philippine Islands. Albatross Expedition, Mindanao Island, 7°47.00’N, 123°31.15’E, 333 m, 9.VIII.1909, 1 20.0 × 17.5 mm ( USNM 128579).
SIZE. — Both of the specimens reported above are the largest known male and female. The male from Guam exceeds the size of the male type, 22.0 × 19.0 mm, from Japan ( Sakai 1969). The female, 20.0 × 17.5 mm, from the Philippines is the largest female reported.
DEPTH AND HABITAT. — Known depth range includes 48- 430 m. Unfortunately, the exact depth where the Guam specimen was captured was not recorded, but it must be relatively shallow, probably less than 48 m, because it was taken by a diver. The Philippine specimen came from the deeper end of the range, 333 m but did not extend the maximum depth for T. cristatipes . Like the other members of this genus, T. cristatipes is not known to carry pieces of camouflage.
DISTRIBUTION. — Previously known from Japan and New Caledonia. Now recorded from the intermediate localities of Guam and the Philippine Islands.
COMPARISON OF THE FAUNAS OF THE MARIANA AND PHILIPPINE ISLANDS
The dynomenid and dromiid faunas of the Mariana and Philippine Islands are listed in Table 1 View TABLE . Dynomene pilumnoides is the only known dynomenid from the Philippines ( McLay 1999). D. hispida , not dealt with herein, has been collected from the northern Mariana Islands of Maug East and Maug North ( Takeda et al. 1994) but so far it has not been collected from Guam. Six dynomenid species are known from the Mariana Islands: D. hispida , D. praedator , D. guamensis n. sp., D. kroppi n. sp., Hirsutodynomene spinosa and Paradynomene tuberculata and six species are also known from Indonesia: D. hispida , D. praedator , D. pilumnoides , H. spinosa , Metadynomene tanensis and P. tuberculata . Since Guam and Indonesia, on either side of the Philippines, share four of the eight species it seems reasonable to expect that these will also be found in the Philippines. Cryptodromia tuberculata and C. fallax were recorded from the Philippines by Cowles (1913) and Ward (1941) respectively. Ward also recorded Cryptodromiopsis bullifera from Gulf of Davao, Mindanao, Philippines. Alcala (1974) reported Dromia dormia from coral reefs near Dumaguete City, Philippines where it preyed upon the crown of thorns starfish ( Acanthaster planci ). New dromiid records for the Philippine Islands include Sphaerodromia nux , Lauridromia indica , Cryptodromia pentagonalis , C. trituberculata and Takedromia cristatipes . In the Philippine Islands a total of 20 dromiid species (in eigth genera) and one dynomenid are now known. New dromiids reported for the first time from Guam include C. hilgendorfi , C. fallax , Cryptodromiopsis unidentata and Takedromia cristatipes . For the Mariana Islands, as a whole, there are now a total of nine dromiid species (in four genera) and six dynomenid species (in three genera) known. A total of 29 dynomenid and dromiid species are reported from the Mariana and Philippine Islands, of which only seven species, all of them dromiids, are shared.
“ Dromia verrucosipes White, 1847 ”, a nomen nudum, was based on specimens purchased from a Mr H. Cuming who obtained them from the Philippine Islands. Subsequently “ D. verrucosipes ” was synonymised with Cryptodromia lateralis ( Gray, 1831) , from Australia, by Miers (1884) and Henderson (1888). As a result, Estampador (1937: 510) listed C. lateralis in his checklist of Philippine decapods. McLay (1993: 168) transferred Dromia lateralis Gray, 1831 to the new genus Stimdromia and showed that the Philippine specimens belonged to an undescribed species of Stimdromia . This species is listed in Table 1 View TABLE as Stimdromia sp.
Cryptodromiopsis unidentata , reported herein, is the only known dromiid from Tonga. No dynomenids are known from this island. Besides Cryptodromia tumida , other dromiids and dynomenids known from Samoa include C. coronata , Dynomene hispida and D. praedator ( McLay 1993, 1999).
CRISTA DENTATA AS A BRACHYURAN CHARACTER Boas (1880) seems to have been the first to use the term “crista dentata” to refer to structures on the inner margin of the mxp3 ischium (or basisischium) of decapods. He used the term indiscriminately, in reference to the mxp3 of both natant and reptant decapods, so that its exact original meaning is unclear. Subsequently, the term crista dentata has been used to refer to a comb-like row of sharp horny teeth arranged along the inner margin of the ischium ( McLaughlin 1980). These teeth are used to grasp food passed to the mxp3 by the chelipeds. The outer ischial margin can be setose but often lacks any tubercles.
According to Scholtz & Richter (1995), the crista dentata is found among all the Eureptantia Scholtz & Richter, 1995 (that is all Reptantia Boas, 1880, except for the Polychelidae Wood- Mason, 1875) and represents an apomorphy of this group. Within the Brachyura the presence of a crista dentata is a plesiomorphic feature of the Dromiacea De Haan, 1833 because it is present in all three families: Homolodromiidae , Dromiidae , and Dynomenidae (although it has been lost in the two species of Acanthodromia ). Among the remaining podotreme crabs, the crista dentata is present only in the Homolidae . The dromiids, dynomenids and some homolids (e.g., Latreillopsis Henderson, 1888 , Homolochunia Doflein, 1904 and Homolomannia Ihle, 1912 ) are the only podotremes with operculiform mxp3 armed with a crista dentata. The homolodromiids and other homolids (e.g., Homola Leach, 1815 , Moloha Barnard, 1947 and Dagnaudus Guinot & Richer de Forges, 1995 ) have pediforme or sub-pediforme mxp3 (see Guinot 1995 and Guinot & Richer de Forges 1995). If we suppose that the Dromiacea, Homoloidea Guinot & Tavares (2001) and the remaining podotremes ( Cyclodorippidae , Cymonomidae , Phyllotymolinidae , Latreilliidae and Raninidae ) shared a common ancestor (with pediforme mxp3 and a crista dentata) then we must assume that the crista dentata has been independently lost several times: among some dynomenids (i.e. Acanthodromia ), and in latreilliids, cyclodorippids, cymonomids, phyllo- tymolinids and raninids. The crista dentata, as found in podotreme crabs, is absent from all of the Eubrachyura. Among these crabs the grasping role of the mxp3, if it is functional, is performed by teeth-like tubercles on the outer margin of the ischium. These teeth-like tubercles, found in the Eubrachyura, are not, as is often assumed, homologous with the crista dentata. Since this term has been used for the exterior tubercles of eubrachyurans, another term is required. A suitable descriptive term for them would be marginal dentata.
UROPODS AND THE ABDOMINAL LOCKING MECHANISM
In brachyurans, the abdomen is reduced and folded ventrally against the sternum. Its role is mainly protective: forming an incubation chamber in females and covering the gonopods in males. Associated with the change in the role of the abdomen from locomotion to protection, has been the evolution of some kinds of mechanism for securing the abdomen out of the way ( Guinot & Bouchard 1998). These authors point out that abdominal locking mechanisms involve some level of coaptation between two independent parts of the body of the crab. They recognized three levels of coaptation: simple juxtaposition, engagement (engrenage) and assemblage (assemblage). These represent different degrees of specialization and it is clear that, for any structures to be recognized as being part of an abdominal locking or retaining mechanism, there must be some kind of coaptation between the abdomen and another part of the body. Comparison of the abdominal locking mechanisms of brachyurans provides some valuable insights into their phylogenetic relationships. While we find only a single mechanism among eubrachyurans (the boutonpression, a coaptation by assemblage that is an apomorphy of this group), the Podotremata show a variety of mechanisms involving structures on either the coxae of p1-p3 or the sternum (coaptations by juxtaposition or engagement). Some podotremes do not seem to have any functional mechanism, not even coaptation by juxtaposition. The variety of mechanisms among podotremes is linked to the presence of uropods, the size of the abdomen and consequently its proximity to the bases of the pereopods. Another factor is the degree of sexual dimorphism in abdomen size. There is a relatively small difference in size between males and females.
In the Dynomenidae , three conditions are found: there are tubercles on sternite 5 in Dynomene and Hirsutodynomene ; in Acanthodromia there are coxal spines on mxp3 and p1-p3 and in Metadynomene there is a small ridge or spine on p2-p3 coxae, but no granules on sternite 5; and in Paradynomene there are both granules on coxae of p2-p3 and a few granules on sternite 5 (but these do not appear to be effective). Although there are small sternal tubercles at the bases of the p2’s in Dynomene , Hirsutodynomene , and Paradynomene , these are not involved in retaining the abdomen. When the abdomen is closed the uropods lie beside these tubercles, but not touching them. There is no locking mechanism so that the abdomen must be held against sternum by muscular tension. At the most, these tubercles can only function to restrict sideways movement of the abdomen. There is no evidence of coaptation of the abdomen, even by juxtaposition, utilizing these tubercles as a holding mechanism. Video-taped aquarium observations of live D. praedator from Hawaii, show that during movement the abdomen is not held tightly against the sternum, and periodically “pumping” movements are made by flicking the abdomen. These abdominal movements are also seen when the crab is stationary and feeding. The function of the sternal tubercles is enigmatic. In Metadynomene and Paradynomene there are small ridges or spines on the coxae of p2-p3, adjacent to the uropods or penultimate segment of the abdomen, but, again there is no evidence of coaptation. In the remaining dynomenid genus, Acanthodromia , an abdominal locking mechanism is present and functional. The abdomen fits closely against the bases of the pereopods and is held in place by spinous coxal projections on the mxp3 and first three pereopods. Acanthodromia shows coaptation by juxtaposition or engagement.
In dynomenids, sternal tubercles may lie beside the uropods, but since the uropods do not project beyond the lateral abdominal margin they cannot lock in front of these tubercles. In some species there is more than one sternal tubercle present and it may be that they are simply part of the external ornamentation of the body, and their presence near the margins of the abdomen are purely coincidental. Whether they have a role in the live animal needs to be verified, because they certainly do not appear to function in dead specimens. The presence of coxal projections in Acanthodromia , Metadynomene and Paradynomene resembles that found in dromiids. However, since the uropods in dynomenids never project beyond the lateral margins of the abdominal segments they cannot be involved in retaining the abdomen as in dromiids.
Sexual dimorphism of the abdomen, sternal tubercles and the uropods varies between the dynomenid genera. In females the abdomen totally covers the sternum and bases of pereopods, extending anteriorly so that the telson covers the bases of mxp3. The male abdomen is smaller, also covering most of the sternum, but not the bases of the pereopods. The lack of strong sexual dimorphism in abdomen size is a plesiomorphic feature of dynomenids. In males and immature females of Dynomene , Hirsutodynomene , and Paradynomene small tubercles are present at the bases of the p2’s, but in mature females these tubercles are usually absent. In all species of Dynomene and Hirsutodynomene the uropods are sexually dimorphic with female uropods always being larger and occupying a greater proportion of the lateral abdominal margin. In Metadynomene the uropods occupy the entire margin between the telson and penultimate abdominal segment while in Paradynomene they fill about half the margin. The uropods are not sexually dimorphic in these two genera. The relative size of uropods in male and female Acanthodromia is not known. Compared to dromiids, dynomenid uropods are much larger, probably retaining the plesiomorphic condition. The larger size of the loosely held abdomen is also closer to the presumed ancestral state, wherein it was used in locomotion.
The greatest diversity of abdominal retaining mechanisms is found among the dromiids. We should firstly note that, compared to dynomenids, the dromiids have much stronger sexual dimorphism of the abdomen. This reflects the narrower sternum in dromiid males. In dynomenids the male and female sterna are about the same width. In dromiids the uropods are also strongly sexually dimorphic. In males and immature females they project laterally, whereas in mature females they do not project but simply fill part of the space between the telson and last abdominal segment, as found in dynomenids. In all cases the abdomen retaining mechanism in dromiids involves structures on the coxae of the first three pereopods, which may act singly or in pairs, but never all three together (see Guinot & Bouchard 1998). In some genera uropods are vestigial [ V] and/or absent [A]: Ascidiophilus Richters, 1880 [A] and Pseudodromia Stimpson, 1858 [ V] (both of which live tightly enclosed in ascidians), Tunedromia McLay, 1993 [A], Epipedodromia André, 1932 [A], Haledromia McLay, 1993 [ V], Exodromidia Stebbing, 1905 [ V or A], Eudromidia Barnard, 1947 [ V], Dromidia Stimpson, 1858 [ V], Austrodromidia McLay, 1993 [ V or A], Barnardromia McLay, 1993 [ V], Speodromia Barnard, 1947 [ V], and Hypoconcha Guérin-Méneville, 1854 [ V or A]. In species of most of these genera, the abdomen is retained by coxal structures held against the abdominal margins. Vestigial or absent uropods must represent the most derived state. In the remaining dromiid genera the uropods are relatively well-developed and often involved in the abdominal locking mechanism.
Coxal abdominal locking mechanisms occur in all dromiacean families. In the Homolodromiidae a retaining mechanism is normally absent, except in Dicranodromia felderi Martin, 1990 , where the telson is held by flanges on coxae of p1. Similarly, amongst dynomenids a functional coxal mechanism is only found in Acanthodromia . In other species or genera in these two families, a retaining mechanism is absent. Amongst dromiids coxal mechanisms are widespread. These involve various structures on the coxae of p1 to p3 meshing with the posterior abdominal segments, uropods and sometimes the telson (see Guinot & Bouchard 1998 for a detailed summary). Evidence of the role of the coxal tubercles and ridges can easily be seen in the coaptation of the posterior abdominal segments with these structures. The most derived abdominal retaining mechanism is found where uropods fit tightly in front of coxal structures on the second pereopods as occurs in species of Dromia . Guinot & Bouchard (1998) call this condition the “full lock system”. The abdomen can only be released when the second pereopods are moved forwards, thereby moving the coxal tubercles posteriorly and releasing the uropods that are locked in front of the tubercles. This is coaptation by assemblage. McLay (1999: 463) argued that the ancestral dromiacean had no retaining mechanism and that the abdomen was simply retained by muscular tension. The simplest explanation of the evolution of these mechanisms is that coxal retaining mechanisms have evolved independently in each of the three families from an ancestor without such a mechanism. Thus the strong sexual dimorphism of the abdomen seen in dromiids is an apomorphy of this group. Similarly, in the Homoloidea De Haan, 1839, we find coxal mechanisms as well as a unique press-button system on sternite four (termed the “homoloid press-button” by Guinot & Bouchard 1998). Thus it is clear that, amongst the Podotremata, many different abdominal holding mechanisms have evolved independently. The bouton-pression of the Eubrachyura, on sternite 5, is an apomorphy of this group, and presumably evolved from an ancestor lacking an abdomen retaining mechanism.
DYNOMENID AND DROMIID GONOPODS
Some general comments about the variation of dynomenid gonopods and their possible role in species recognition are appropriate here. The first gonopod is a partially rolled tube that forms a tube only distally. At the tip there is an aperture, through which the sperm must pass, with a soft medial plate on one side and finally a “fence” of filiform setae surrounding the tip. Scanning electron microscope pictures of the tip of the first gonopods of eight species (in four genera) show no consistent variation between species or genera (see McLay 1999: 459, figs 12-14). The second gonopod is long, needle-like and armed with a single row of spines towards the tip. The main sources of variation on the second gonopod are the number of terminal and subterminal spines, their disposition to each other and the direction in which they point. The number of terminal spines ranges from one to three (curved or straight), while the number of subterminal spines ranges from 4 to 24. Species of Dynomene tend to have a smaller number of gonopod spines (4 to 15) than is found in other genera. The direction and disposition of the subterminal spines, depends upon their arrangement along the gonopod shaft and whether they follow a sinuous or spiral path.
An important distinction needs to be made between those parts of the gonopods, which come into contact with the female, and those parts that do not. Sexual selection could be expected to operate on the first but not necessarily on the second, unless they are somehow linked to the first. When the second gonopod is inserted inside the first, the only parts that can come into contact with the female are the tips of the first and second gonopods. The tip of the second gonopod can extend out of the aperture of the first but only for a short distance. With the first gonopod closely applied to the spermathecal aperture, the second gonopod can enter the spermathecal opening for a short distance. This means that only the spines near the tip of the gonopod can interact with the female aperture: these are the terminal spines and those subterminal spines nearby. Therefore only variation in these spines can be explained by sexual selection. However, I suggest that in the copulatory position all dynomenid gonopods will appear to be nearly the same. Most of the variation in gonopod structure occurs in parts of the second gonopod that cannot be in contact with the female. Therefore sexual selection cannot be invoked as an explanation of the variation in dynomenid second gonopods. If the subterminal spines have any function at all it must be in the area of facilitating sperm transfer or cleaning the pathway followed by the sperm. The mechanism of sperm transfer in dynomenids is poorly understood, as it is in all dromiacean crabs (see McLay 2001 for further discussion of this point).
Dromiid males have similar gonopods to dynomenids, supporting the sister group relationship of these two families. The first gonopod is a poorly formed conduit (as in dynomenids) and the second is longer than the first, needle-like and normally lacks a row of spines. An interesting point, overlooked by McLay (1991, 1993), is that the gonopods of the dromiid genus, Sphaerodromia , are almost identical to those of dynomenids. In S. ducoussoi McLay, 1991 , for example, the first gonopod has a setose tip with a flexible medial plate, as in dynomenids, but also has a blunt lateral knob on the opposite side. In between these lies the small aperture through which the second gonopod emerges. When the gonopods are brought together with the female spermathecal aperture, the blunt knob engages with a depression on the sternum and the plate fits closely alongside the aperture. The second gonopod has a row of 20 small inset spines similar to that found in Hirsutodynomene (see Fig. 10 View FIG ). Similar structures are found in S. nux Alcock, 1899 (see 24.0 × 23.3 mm, male specimen MNHN-B 6922).
The genus Sphaerodromia is regarded, for several other reasons, as being closest to the dromiid ancestral condition (see McLay 1993: 127). All other dromiid genera retain the setose tip, but lack the soft medial plate and lateral knob on the first gonopod, and the shaft of the second gonopod lacks spines. This clearly represents the apomorphic condition amongst the Dromiidae . The Homolodromiidae lack a medial plate on the first gonopod and the second is without spines. It maybe that the medial plate and spinous second gonopod are linked with the very short sternal sutures 7/ 8 in dynomenids and Sphaerodromia . In these animals the angle of the male gonopods, during mating, would have to be greater due to
A
the more posterior placement of the spermathecal openings, and the plate and spines may ensure better sperm transfer by preventing leakage.
What do the gonopods of dromiaceans tell us about their phylogenetic relationships? The homolodromiids seem to have retained the ancestral condition (no medial plate on the first gonopod and no spines on the second) while the ancestor of the dynomenids + dromiids evolved new structures (plate + spinous second gonopod). We have to assume that these new structures were secondarily lost in advanced dromiids, perhaps linked to the evolution of longer sternal sutures 7/8 and more anterior position of the spermathecal openings representing the derived condition. These arguments are not unreasonable and are consistent with other characters supporting the hypothesis of McLay (1999).
Among the homolodromiids, Guinot (1995) found broken-off tips of the male first gonopods in the spermathecal orifices of some females. For this to occur the second gonopod must be longer than the first and be able to enter the openings. In dynomenids and dromiids the second gonopod can also extend out of the tip of the first, and potentially enter the spermathecal aperture of the female, but broken off pieces have never been found in any species of these families. It is unclear whether these broken pieces of the G 2 in homolodromiids are accidental (representing an injury to both parties) or normal and have some role such as dislodging or displacing the sperm of earlier matings. Alternatively they may be some novel kind of “sperm plug”.
| V |
Royal British Columbia Museum - Herbarium |
| R |
Departamento de Geologia, Universidad de Chile |
| GUM |
Glasgow University Museum (Hunter Museum) |
| T |
Tavera, Department of Geology and Geophysics |
| VI |
Mykotektet, National Veterinary Institute |
| ZRC |
Zoological Reference Collection, National University of Singapore |
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
Genus |
Cryptodromiopsis plumosa ( Lewinsohn, 1984 )
| Mclay, Colin L. 2001 |
Cryptodromiopsis unidentata
| MCLAY C. L. 1993: 192 |
Takedromia cristatipes
| MCLAY C. L. 1993: 212 |
Dromidiopsis plumosa
| MCLAY C. L. 1991: 470 |
| LEWINSOHN C. 1984: 104 |
Cryptodromia cristatipes
| SAKAI T. 1969: 245 |
Dromia unidentata Rüppell, 1830: 16
| LEWINSOHN C. 1984: 107 |
| RUPPELL E. 1830: 16 |