Cassida (Tylocentra) rothschildi Spaeth, 1922
|
publication ID |
https://doi.org/10.5281/zenodo.5339545 |
|
DOI |
https://doi.org/10.5281/zenodo.5879621 |
|
persistent identifier |
https://treatment.plazi.org/id/03FDE60C-FFEA-FFF4-894F-44A6CE2FFAE2 |
|
treatment provided by |
Felipe |
|
scientific name |
Cassida (Tylocentra) rothschildi Spaeth, 1922 |
| status |
|
Cassida (Tylocentra) rothschildi Spaeth, 1922 View in CoL
( Figs. 1–27 View Figs View Figs View Figs View Figs View Figs View Figs View Figs )
Cassida Rothschildi Spaeth, 1922: 1002 View in CoL .
Cassida rothschildi: BOROWIEC (1999) View in CoL : 277 (catalogue); BOROWIEC & SEKERKA (2010): 377 (catalogue).
Type locality. Lasami in Randile region (northern Kenya, close to Turkana Lake).
Type material. HOLOTYPE: ‘Afrique Orient. Angl. / Lesammise Rendile / Maurice de Rothschild 1906 // TYPE’ (preserved in Muséum National d’Histoire Naturelle, Paris, France).
Material examined (94 spec.). YEMEN: SOCOTRA ISLAND: Firmihin plato, Dracena tree forest , 12°28′465′′N 54°00′89830′′E, 22.-25.vi.2009, 21 spec. and 2 larvae, V. Hula leg. (7 spec. and 2 larvae in DBET, rest in JBCB); Dixam plateau, Firmihin ( Dracaena forest ), 12°28.6′N 54°01.1′E, 490 m, 15.-16.xi.2010, 28 spec., J. Bezděk leg. ( JBCB); GoogleMaps same data, but L. Purchart leg., 7 spec. ( JBCB); GoogleMaps same data, but J. Hájek leg., 8 spec. ( NMPC); GoogleMaps same data, but J. Batelka leg., 3 spec. ( JBCP); GoogleMaps Diksam plateau, 12°31′24′′ N 53°58′29′′E, 850-920 m, 5.ii.2010, 20 spec., L. Purchart & J. Vybíral leg. ( JBCB, 10 spec. in LSCL); GoogleMaps Wadi Zirik, 12°29.584′N 53°59.475′E, 12.vi.2010, 2 spec., V. Hula & J. Niedobová leg. ( JBCB); GoogleMaps Noged, Farmihin, Steroh , Wadi , 12°24′26′′N 54°08′40′′E, 24.x.2000, 1 spec., T. Van Harten leg. ( NHMB). GoogleMaps KENYA: Elsamere, 7.iv.1998, on Lycium shawii , 2 spec., ABD ( DBET). SAUDI ARABIA: BAC Camp , Khamis Mushayt , 2000 m, 17.-18.iv.1976, 1 spec., Wittmer & Buettiker leg. ( NHMB). SUDAN: Kassala, Abend Pass, 5.xii.1962, 1 spec., Linnavuori leg. ( DBET).
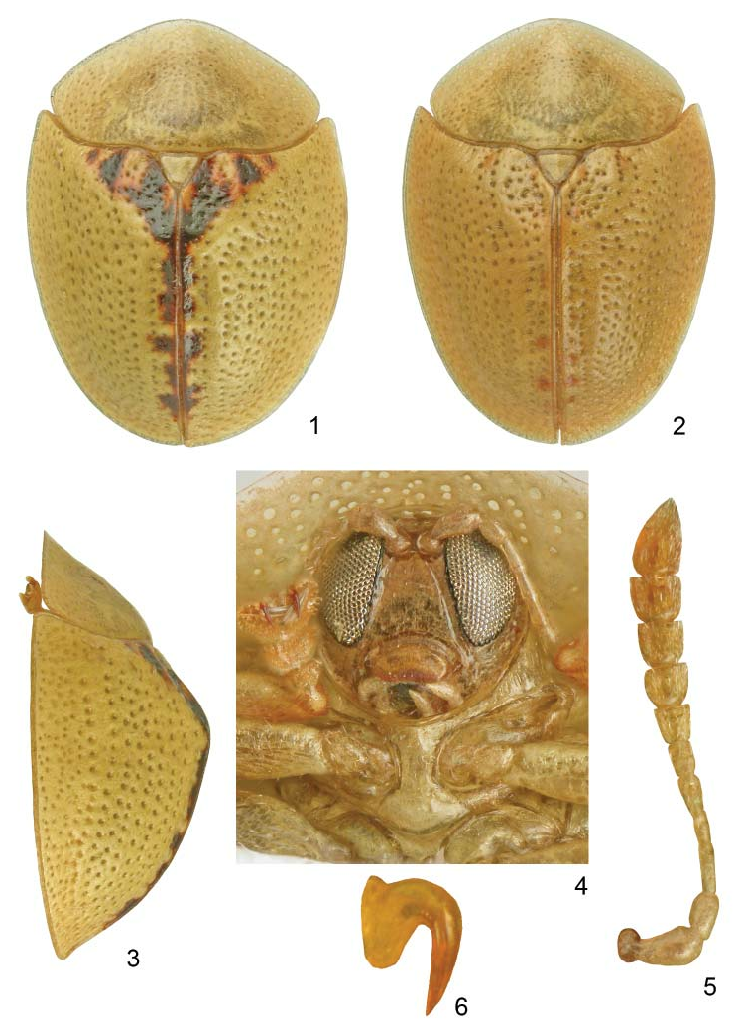
Redescription of imago. Length: 3.8–4.4 mm, width: 2.9–3.3 mm, length of pronotum: 1.4–1.5 mm, width of pronotum: 2.4–2.8 mm, length/width ratio: 1.31–1.35, width/length ratio of pronotum: 1.72–1.83. Body broadly oval, sides distinctly converging posterad ( Figs. 1, 2 View Figs ).
Dorsum yellow (in fresh specimens green), basal impression of elytra and suture usually marked with reddish to brown spots. In palest specimens sides of basal impressions and close to scutellum, apex of disc, and suture in posterior half with few small, reddish spots ( Fig. 2 View Figs ). In darkest specimens almost whole basal impression and along suture with irregular, dark brown spots, often margined with red ( Fig. 1 View Figs ). Between palest and darkest form all intermediate forms were observed. Ventrites, legs and antennae uniformly yellow.
Pronotum ellipsoidal, with subangulate sides, no basal corners, widest approximately in middle. Disc indistinctly bordered from explanate margin, moderately convex, with small but dense punctation, distance between punctures smaller than puncture diameter, interspaces shiny. Explanate margin with dense but very shallow punctation, appears rather irregular than punctate, transparent with honeycomb structure.
Scutellum triangular, impunctate, without transverse sulci. Base of elytra only slightly wider than pronotum, humeral angles distinctly protruding anteriad, angulate. Disc strongly convex, subangulate in profile ( Fig. 3 View Figs ). Postscutellar impression well marked, lateral impressions not present. Punctation arranged in regular rows, only postscutellar impression with partly irregular punctures. Rows not impressed, punctures moderately coarse, distance between punctures mostly wider than puncture diameter. Marginal row distinct, its punctures not coarser than punctures in lateral rows. Intervals flat, 1.5–2.0 times wider than rows, surface microreticulate but shiny. Marginal interval distinct, in anterior half twice wider than lateral intervals, without transverse folds. Explanate margins strongly declivous, in widest part approximately twice narrower than each elytron, their surface sparsely irregularly punctate, punctures approximately twice smaller than in rows.
Eyes large, gena almost obsolete. Clypeus broad, approximately 1.4 times as wide as long. Clypeal lines distinct, running close to margin of eyes and converging in obtuse triangle. Surface of clypeal plate flat, shiny, with several small, setose punctures. Labrum without median emargination ( Fig. 4 View Figs ). Antennae stout, antennomeres IX and X approximately 1.2 times as wide as long, length ratio of antennomeres: 100:65:72:43:57:50:52:57:64:66:118, antennomere II slightly shorter than antennomere III, antennomere III approximately 1.6 times as long as antennomere IV ( Fig. 5 View Figs ).
Prosternal collar very short, forms narrow transparent margin. Prosternal process in middle slightly narrower than mid coxa, strongly expanded apically, rhomboidal apex twice wider than intercoxal space. Area between coxae flat or shallowly impressed, without special sculpture, shiny; rhomboidal apex flat or slightly convex, impunctate ( Fig. 4 View Figs ).
Legs stout, tarsi moderately elongate, claws segment not extending behind marginal setae. Claws simple, on inner side with distinct micropecten ( Fig. 6 View Figs ).
Description of mature larva. Measurements (n = 2). Length without head, from anterior border of pronotum to base of supra-anal processes: 4.10–4.30 mm; width of metathorax, without lateral scoli: 1.90–2.15 mm. Length of supra-anal processes, from base on abdominal segment IX to the top of processes: 1.55–1.70 mm. Width of head: 0.76–0.78 mm.
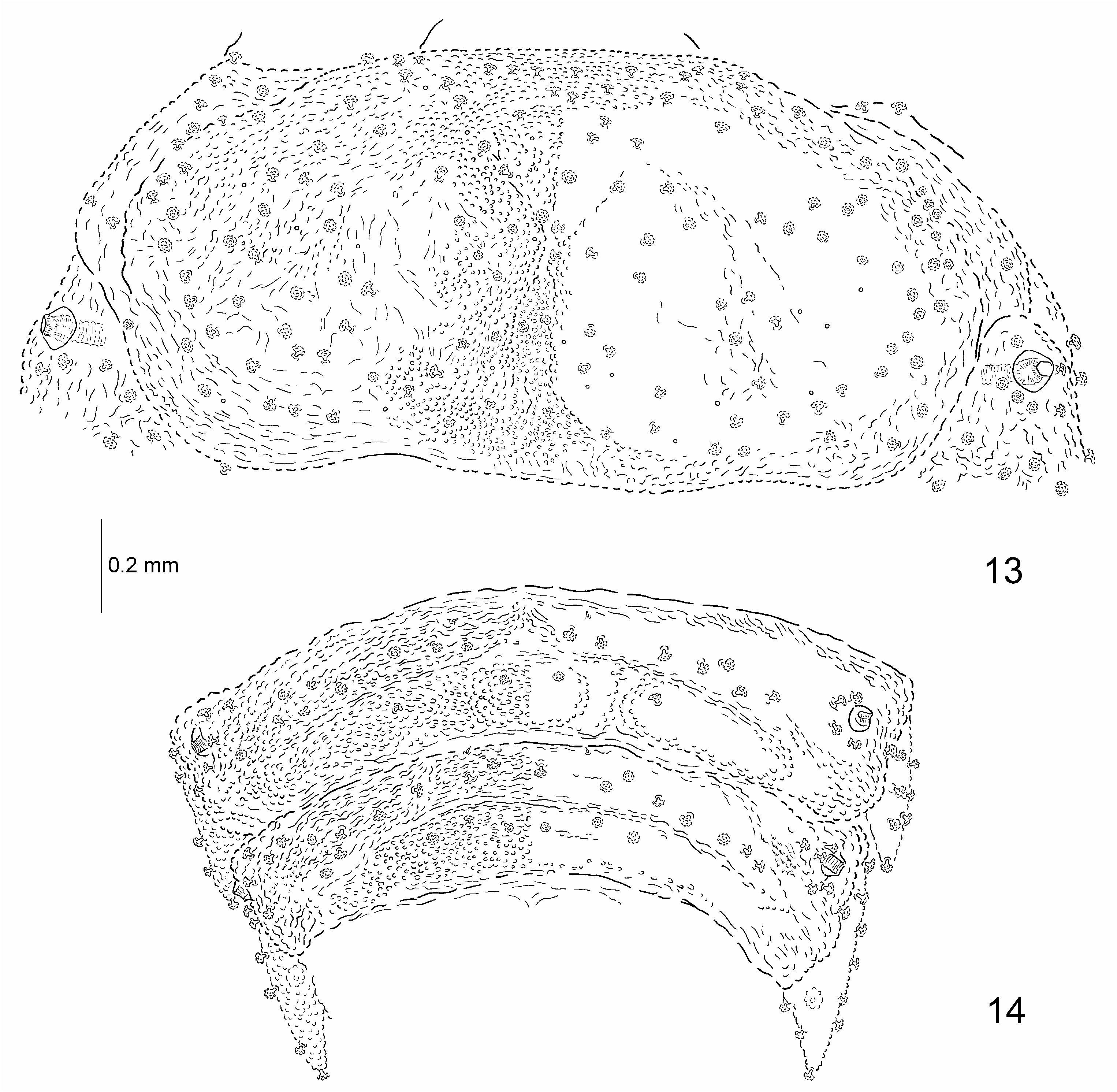
Body elongate-oval, widest across meso- and metathorax, narrowed posteriorly ( Figs. 7–9 View Figs ). Anterior part of body convex ( Fig. 10 View Figs ). Body of larvae preserved in alcohol yellowish with brown basal half of supra-anal processes.
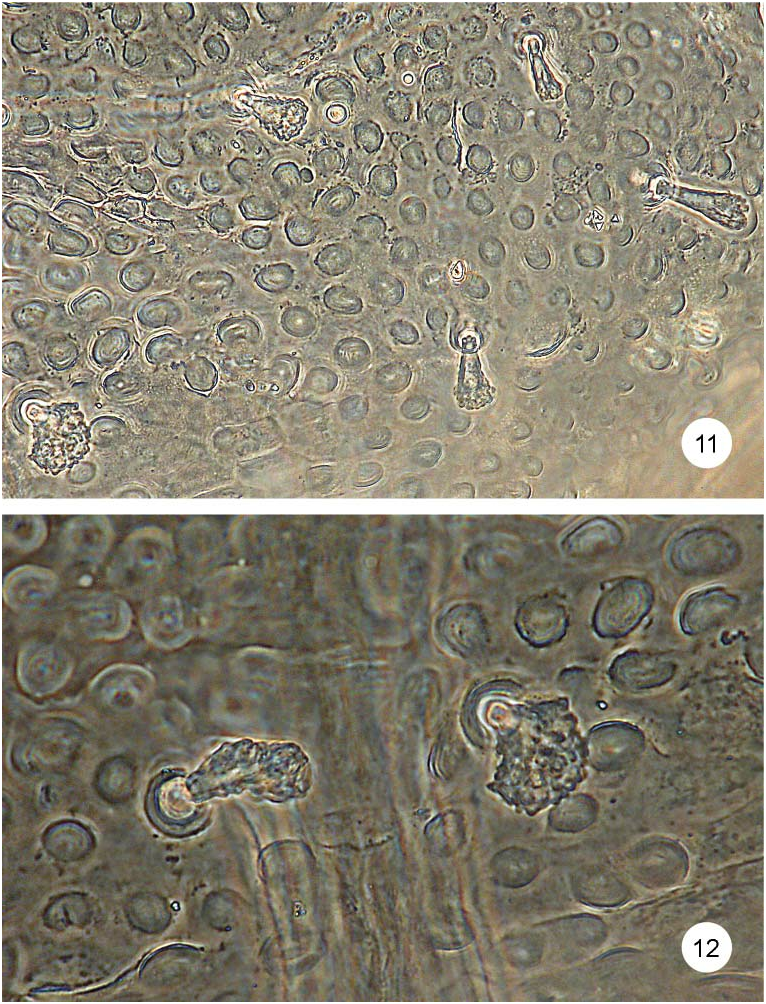
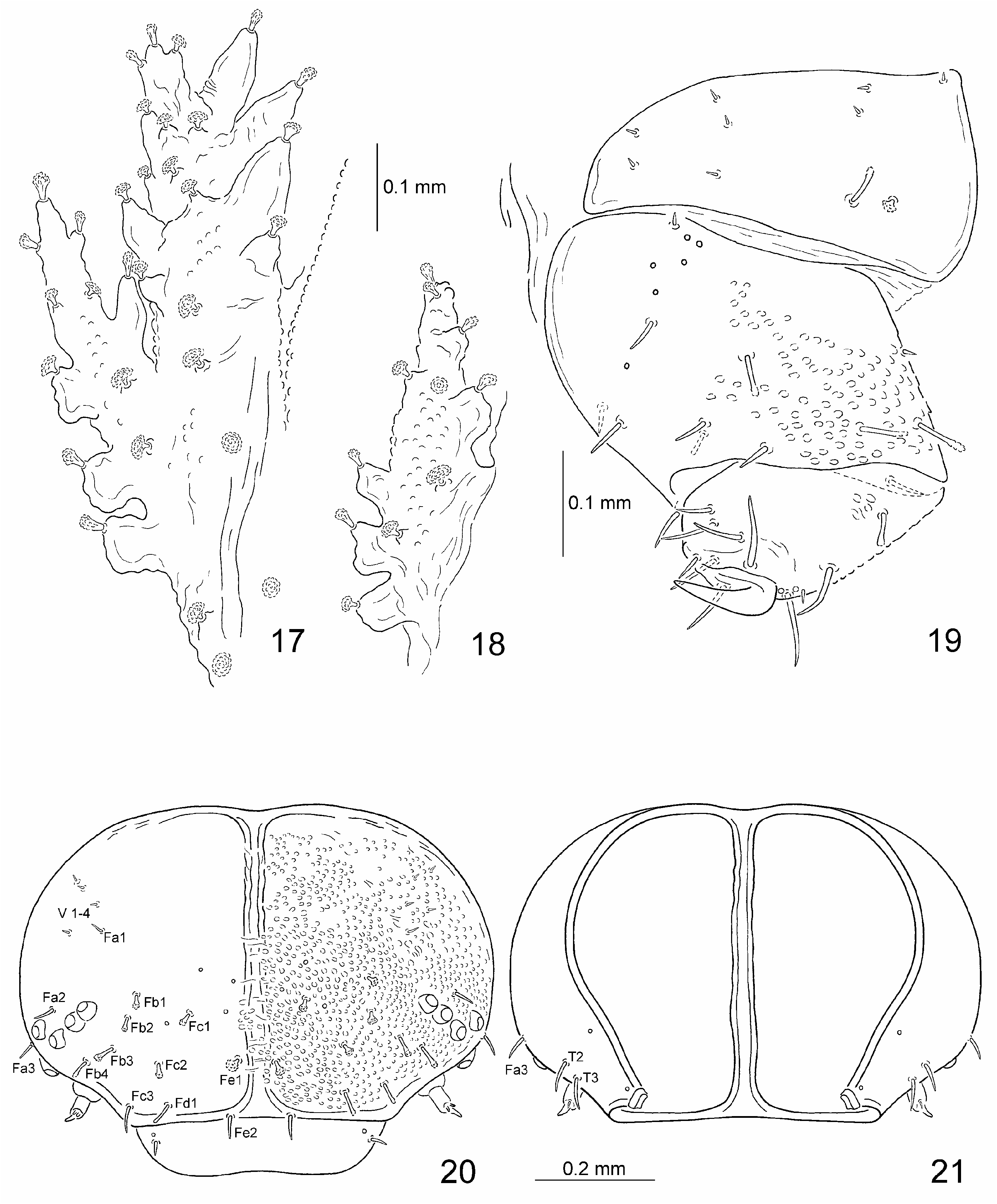
Body with 16 pairs of lateral scoli and a single pair of supra-anal processes ( Figs. 7, 8 View Figs ). Lateral scoli short, stout, conical, approximately same size. All scoli covered with numerous cauliflower-shaped sensilla ( Figs. 17, 18 View Figs ). Scoli of thorax with short, stout lateral processes, each armed apically with cauliflower-shaped sensillum, scoli of abdomen simple without lateral processes. Scoli of first two pairs placed very close to each other ( Fig. 17 View Figs ). Scoli of thorax directed anteriorly, of abdomen posteriorly. Supra-anal processes long, approximately as long as half of body.
Dorsal and ventral side of the body distinctly granulate ( Figs. 13–16 View Figs View Figs ). Minute setae at anterior border of each tergites and sternites. Tergites covered with cauliflower-shaped sensilla ( Figs. 13, 14 View Figs ). Pro-, meso- and metasternum and first two abdominal sternites with pointed setae medially and cauliflower-shaped sensilla laterally ( Fig. 15 View Figs ). Remaining abdominal sternites with cauliflower-shaped sensilla ( Fig. 16 View Figs ).
Pronotum with numerous cauliflower-shaped sensilla distributed regularly ( Fig. 13 View Figs ). Meso-, metanotum and abdominal tergites with two irregular rows of numerous cauliflower-shaped sensilla running across segment and two minute setae at anterior border medially ( Fig. 14 View Figs ).
Two minute setae at anterior border of pro-, meso- and metasternum. Pro-, meso- and metasternum also with two groups of around four setae antero-medially and pair of setae postero-medially ( Fig. 15 View Figs ). Two minute setae at anterior border of each abdominal sternite ( Fig. 16 View Figs ). First two abdominal sternites with numerous setae medially and numerous cauliflower-shaped sensilla laterally. Abdominal sternites III-VIII with numerous cauliflowershaped sensilla distributed regularly.
Anal turret distinctly consists of two segments.
Nine pairs of distinctly elevated spiracles: one on thorax and eight on abdomen. Diameter of spiracles slightly decreasing posterad, spiracles of abdominal segment eight smallest.
Head well sclerotised, hypognathous, retracted into pronotum ( Figs. 8, 10 View Figs ). Median suture complete, connected with fronto-clypeal suture ( Figs. 20, 21 View Figs ). Clypeus distinct, wider than long, with one seta and one campaniform sensilla on each lateral side ( Fig. 20 View Figs ). Frontal and epicranial suture absent, fronto-clypeal and clypeo-labral suture well developed.
Six stemmata on each side of head.
Frontal side of head with four small, vertical, pointed setae (V 1–4); five frontal rows of cauliflower-shaped sensilla ( Figs 11, 12 View Figs ) and setae: row Fa with three sensilla, Fb with four sensilla, Fc with three sensilla, Fd with single sensillum, Fe with two sensilla; and three campaniform sensilla above sensilla Fc1 and Fe1 ( Fig. 20 View Figs ). Temporal side of head with two setae (T 2, T3) and two campaniform sensilla ( Fig. 21 View Figs ).
Antennae dimerous, set in membranous ring ( Fig. 26 View Figs ). Antennomere I transverse, wider than antennomere II. Antennomere II stout, longer than wide, with small seta and a group of three peg-like sensilla at the apex: one prominent (sensory appendix) and two smaller.
Labrum wider than long, anterior margin not emarginate ( Figs. 22, 23 View Figs ). Anterior margin with six stout setae medially and two short setae on each side. Dorsally: four long setae placed in the middle in one row running across width, two setae close to anterior margin, and two pairs of campaniform sensilla medially. Mid part of ventral surface (epipharyngeal area) with pair of small setae, and two pairs of campaniform sensilla. Numerous small spines medially and on each lateral side.
Mandibles heavily sclerotised, palmate, with five apical teeth in one row and one tooth slightly moved back. Two setae and two campaniform sensilla at base dorsally ( Figs. 24, 25 View Figs ).
Maxillae and labium connate ( Fig. 27 View Figs ). Each stipes (st) with two long setae. Palpifer (pp) with two setae and two campaniform sensilla ventrally and with numerous spines dorsally. Mala (mal) not distinctly bordered from palpifer, bearing six long pointed setae, one long blunt seta, and one short blunt seta (or peg like sensilla?). Maxillary palp one-segmented with three pointed setae, one blunt seta (digitiform sensillum – ds), two campaniform sensilla on sides, and a group of twelve small peg-like sensilla at the apex. Labial palp (lp) one-segmented with group of nine small peg-like sensilla at apex and one campaniform sensillum below apex. Hypopharynx (hyp) covered with numerous spines, and with four campaniform sensilla at base. Prementum (pre) with two long setae and four campaniform sensilla. Postmentum (post) with four setae.
Legs stout, consist of three segments: coxa, femur and tibiotarsus ( Fig. 19 View Figs ). Internal side of coxa with setae arranged in three groups: first group with two short setae (placed close to border between coxa and body); second with three short setae; third with three short setae, and with one elongate and one short cauliflower-shaped sensillum. Femur with eleven moderately long pointed or blunt setae and one short pointed seta placed dorsally close to the base. Basally on internal side of femur a group of five campaniform sensilla and one short pointed seta; at base ventrally one campaniform sensillum. Tibiotarsus apically with heavily sclerotised, curved, single and simple claw armed basally with a pointed seta. Claw and pointed seta surrounded by a complex of six long pointed setae. Tibiotarsus also with three long setae dorsally and two campaniform sensilla and small seta above claw.
Diagnosis of larva. Mature larva of C. rotschildi is in general body shape very similar to the larva of another Tylocentra Reitter, 1926 species – C. turcmenica ( Weise, 1892) described by MEDVEDEV & MATYS (1975). The description is superficial and we found only one distinctive character: body length. Mature larva of C. turcmenica is approximately 6 mm long while larva of C. rotschildi at most 4.3 mm, and it is correlated with body length of imagines: 5.0– 6.5 mm in C. turcmenica , 3.8–4.4 mm in C. rotschildi . Both larvae differ from the typical larva of the genus Cassida in very short and simple scoli and convex anterior part of body (ŚWIĘTOJAŃSKA 2009). In these characters Tylocentra is at first glance similar to larvae of Oxylepus Desbrochers, 1884 and Ischyronota Weise, 1891 but Ischyronota species distinctly differ in completely lacking thoracic and more or less reduced abdominal scoli, and Oxylepus larva differs in short supra-anal processes. In our opinion, the similarity is an effect of evolutionary parallelism correlated with feeding on saline plants.
Host plant. Solanaceae : Lycium socotranum Wagn. & Vierh. (all specimens from Socotra were beated from L. socotranum – V. Hula, J. Bezděk & L. Purchart 2010 observ.), Lycium shawii Roem. et Schult. (based on label data from specimen collected in Kenya: Elsamere).
Distribution. Kenya ( SPAETH 1922, present paper), Saudi Arabia, Sudan and Yemen ( BOROWIEC 1999, present paper). BOROWIEC (1999) recorded it generally from Saudi Arabia and Sudan based on unpublished data, in this paper detailed data are given. First record from Socotra Island. Comments. BOROWIEC (1999) placed C. rothschildi within the subgenus Tylocentra . This placement is now supported by the structure of larva described in this paper and by association with plants of the family Solanaceae , especially various Lycium species.
Cassida rothschildi is the only member of the subgenus Tylocentra known from Africa south of Sahara. The most related species is C. pellegrini Marseul, 1868 recorded from Cyprus, Israel, Lebanon, Saudi Arabia and Tunisia ( SEKERKA & BOROWIEC 2011). Both taxa belong to the group of species with regularly punctate lateral rows on elytral disc but C. pellegrini distinctly differs in more elongate body and less convex elytral disc (see figs. 3 and 4 in SEKERKA & BOROWIEC 2011). Cassida rothschildi is the smallest member of the subgenus with body length below 4.5 mm; other species usually have length above 4.7 mm, although the smallest specimens of C. pellegrini are 4.5 mm in length.
Based on the structure of larva of C. turcmenica Weise, 1892 , MEDVEDEV (1982) raised the subgenus Tylocentra to the genus rank, but he did so without discussion and only noted ‘traditionally it [ Tylocentra ] was included in Cassida as subgenus but study of larva showed that it should be raised to genus, although imago only indistinctly differs from members of other Cassida ’. Larva of C. rothschildi is very similar to larva of C. turcmenica and has the same unique characters – strongly reduced lateral scoli and quite convex body. In other characters it is very similar to many other Cassida species of various subgenera, and reduction of scoli and body convexity is, in our opinion, distinctly correlated with feeding on semisucculent, saline–habitat plants. The tendency to scoli reduction and body convexity was observed also in other Cassidini genera associated with saline plants, e.g. Ischyronota spp. and Oxylepus deflexicollis ( Boheman, 1862) ( BORDY 2000; ŚWIĘTOJAŃSKA & BOROWIEC 2007). Imagines of Tylocentra members have no unique characters, although they form monophyletic and morphologically and biologically coherent group ( BOROWIEC 2007). However, treating it as a separate genus based only on homoplastic larval characters is unjustifed.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cassidinae |
|
Genus |
Cassida (Tylocentra) rothschildi Spaeth, 1922
| Świętojańska, Jolanta & Borowiec, Lech 2012 |
Cassida Rothschildi
| BOROWIEC L. & SEKERKA L. 2010: 377 |
| BOROWIEC L. 1999: 277 |
| SPAETH F. 1922: 1002 |