Macrolycus Waterhouse, 1878
|
publication ID |
https://doi.org/ 10.5281/zenodo.280378 |
|
DOI |
https://doi.org/10.5281/zenodo.6172163 |
|
persistent identifier |
https://treatment.plazi.org/id/03FD486F-FFFE-5452-FF3C-D472FA6B244B |
|
treatment provided by |
Plazi |
|
scientific name |
Macrolycus Waterhouse, 1878 |
| status |
|
Macrolycus Waterhouse, 1878: 96 .
Type species Macrolycus bowringi Waterhouse, 1878 (= Macrolycus coccineus Waterhouse, 1878 nom. nud.; monotypic, by original designation).
Cerceros Kraatz, 1879: 126; Bourgeois, 1882: xlvi.
Type species Cerceros pectinicornis Kraatz, 1879 (by original designation).
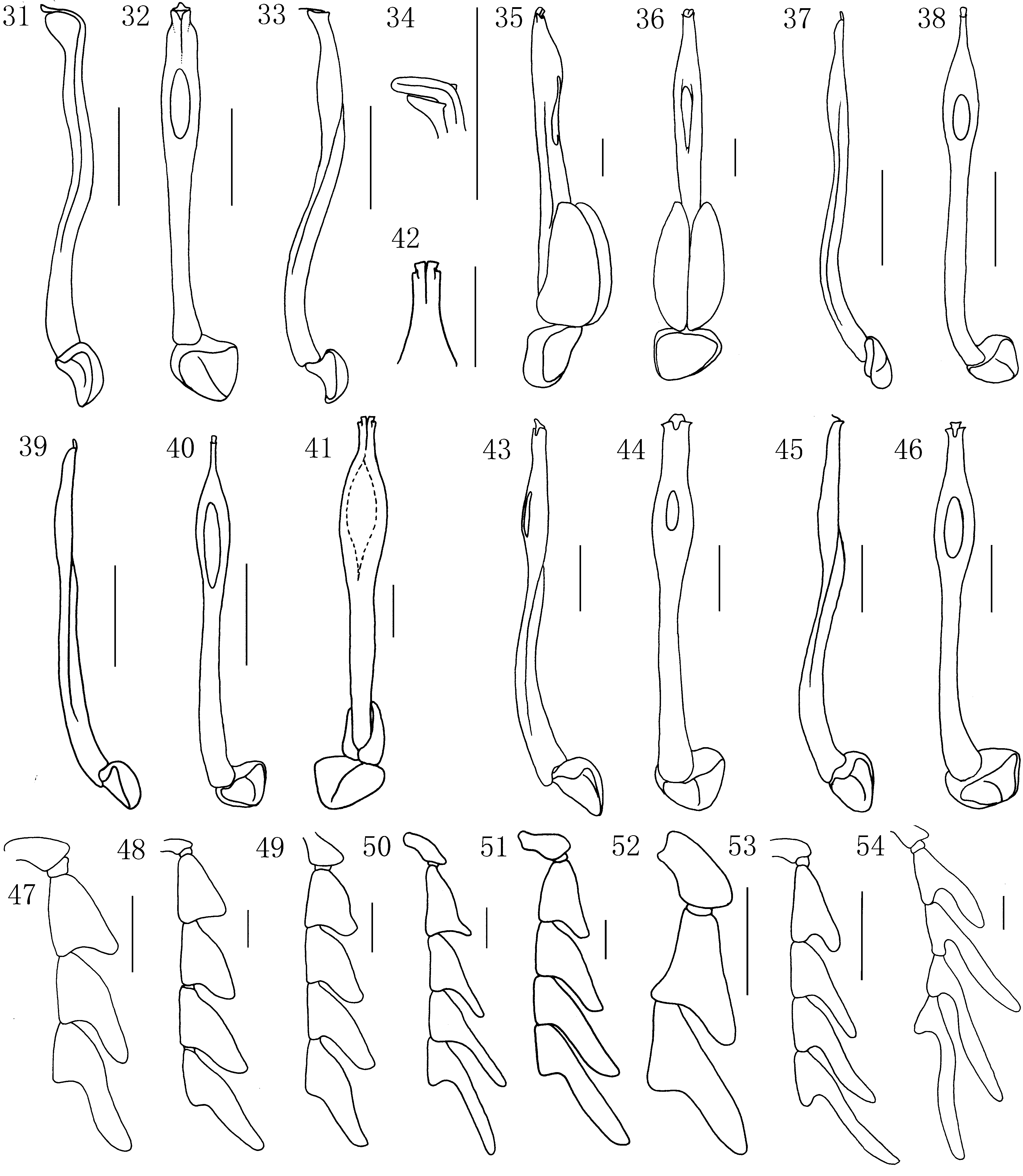
Diagnostic description. Body small to medium sized, parallel-sided to slightly widened posteriorly, dorsoventrally flattened. All species brightly coloured, pronotum and elytra uniformly dark red to orange yellow, or pronotum black and elytra red. Vestiture sparse to dense, short, decumbent or suberect. Head small, transverse, partially concealed by pronotum, hypognathous without rostrum ( Fig. 2 View FIGURES 2 – 22 ). Vertex flat, with coronal suture reaching to the posterior edge of cranium. Hind margin of ventral closure strongly concave, with paired cervical sclerites attached ( Fig. 2 View FIGURES 2 – 22 ). Tentorium with two slender sclerotized ventral arms arising from posterior tentorial pits. Antennal tubercles flat to prominent. Subantennal suture complete. Antennal cavities heart-shaped, moderately approximate, with small process at the anterior-lateral margin. Antennae with eleven densely pubescent antennomeres, acutely serrate to flabellate in males ( Fig. 6 View FIGURES 2 – 22 ), shortly serrate in females ( Fig. 7 View FIGURES 2 – 22 ), reaching to half or two-thirds elytral length when inclined. Scapus robust, pear shaped; pedicel small, transverse, with two minute acute thorns; antennomeres 3–10 triangular or lamellate. Lamellae of male antennae from antennomere 3 or 4 to 7 prolonged gradually, following antennomeres subequal in length, antennomere 11 simple, slender ( Fig. 6 View FIGURES 2 – 22 ). Mouth opening broad, situated ventrally ( Fig. 2 View FIGURES 2 – 22 ). Eyes small, hemispherically prominent, with fine facets, eye diameter as long as half of frontal interocular distance. Anterior margin of clypeus almost straight, fronto-clypeal suture absent. Labrum transverse, with long setae at anterior margin; hypopharynx reflexed laterally, with hypopharyngeal sclerite rigidly attached to labrum ( Fig. 3 View FIGURES 2 – 22 ). Mandibles elongate, slender, moderately to strongly curved, inner margin simple, with one dent or ridge ( Fig. 4 View FIGURES 2 – 22 ). Maxillae with long setose galea; maxillary palpi 4-segmented, palpomeres 1–3 cylindrical, moderately widened at apex, apical palpomere slightly or much wider than long, flattened, securiform ( Fig. 5 View FIGURES 2 – 22 ). Prementum peach-like, mentum transverse; labial palpi 3-segmented, palpomeres 1–2 cylindrical, much shorter and narrower than apical one, palpomere 3 flattened, securiform ( Fig. 9 View FIGURES 2 – 22 ). Pronotum transverse, elevated laterally, with sharp median longitudinal carina in frontal third to half of midline, hind part of median carina absent or inconspicuous, sometimes carina becoming weak longitudinal depression posteriorly ( Fig. 10 View FIGURES 2 – 22 ), more or less apparent vestiges of lateral carinae at lateral margins, lateral carinae always short, never reaching midline. Anterior margin almost straight or projecting forward; hind edge bisinuate; lateral edges parallel-sided, widened posteriorly or curved. Anterior angles obtuse or acute, posterior angles acutely prominent. Prosternum transverse, narrow, prosternal process Y-shaped, with procoxal cavity broadly opened posteriorly ( Fig. 11 View FIGURES 2 – 22 ). Scutellum parallel-sided or narrower posteriorly, straight or emarginate at apex ( Fig. 8 View FIGURES 2 – 22 ). Elytra flat, densely pubescent, subparallel, seldom apparently widened backwards; each elytron with four longitudinal costae, costa 3 sometimes inconspicuous; costal interspaces irregularly punctured, without regular transverse costae; sometimes with irregular cells formed by fine, often interrupted costae. Hind wings well-developed, wing venations uniform, wedge cell absent ( Fig. 17 View FIGURES 2 – 22 ). Anterior margin of mesoventrite concave ( Fig. 13 View FIGURES 2 – 22 ). Mesocoxal cavities well-separated, laterally closed ( Fig. 13 View FIGURES 2 – 22 ); metaventrite broad, with median discrimen reaching to two thirds of posterior part ( Fig. 13 View FIGURES 2 – 22 ); metendosternite leaflike, with longitudinal and transverse suture, lateral arms minute to short ( Fig. 14 View FIGURES 2 – 22 ). Legs slender, compressed, flexible ( Figs 15, 16 View FIGURES 2 – 22 ). Procoxae and mesocoxae globular, metacoxae transverse, apex of coxa with tuft of thorn-like setae. Femora slender, tibia slightly curved, gradually widened distad, with pair of calcars. Tarsi pentamerous; tarsomeres 1–4 with membranous euplantulae; claws slender, bifid at apex. Abdomen flat, slightly sclerotized, with 8 (male) or 7 (female) visible sternites loosely connected by membranes ( Fig. 18 View FIGURES 2 – 22 ); completely covered by elytra in male, but in some females with exposed terminal abdominal segments. Abdominal spiracles from abdominal segment 2 located on sternites. Male terminal abdominal segments as figured ( Figs 18, 19 View FIGURES 2 – 22 ). Median lobe of male genitalia long, slender, nearly straight or curved basally, often with lateral rib and variously modified apex. Subapical part sometimes inflated, with more or less broad ventral opening. Paramerae absent in most species, if present, robust, shell-like or slender apically. Phallobase simple, uniformly hooded, with median longitudinal carina ( Figs 31–46 View FIGURES 31 – 54 ). Female terminal tergite simple ( Fig. 21 View FIGURES 2 – 22 ), sternite 8 with short spiculum ventrale ( Fig. 20 View FIGURES 2 – 22 ). Female genitalia wide, with short rounded styli, coxites short, valvifer slender ( Fig. 22 View FIGURES 2 – 22 ).
Sexual dimorphism. The sexual dimorphism is limited to the relative body size and the shape of pronotum and antennae. Generally, in a series of specimens representing the same species, the largest specimens are females, and the smallest ones are males. One exception is M. atronotatus , in which the female bears much shorter elytra than
male. The female pronotum is relatively broader, sometimes with more obtuse anterior angles and rounded anterior margin. The male antennae are always at least shortly flabellate with more or less prolonged lamellae from the antennomere 3 or 4, and the female antennae are serrate. This difference becomes less conspicuous in some largebodied species as e.g. M. jeanvoinei , since their males have quite short lamellae on antennomeres 3–10, which are almost triangular in shape.
Variability. Similarly with other net-winged beetles, the body shape is quite variable and the definition solely based on the shape of pronotum, strength of costae or presence of reticulate structures in the elytral interspaces may lead to false conclusions on the species limits. The elytral costae may play an adaptive function as strengthening structure, and their multiple origins in different lineages were demonstrated by Bocak et al. (2008). Fine costae can be found in elytral interspaces of M. yunnanus and M. multicostatus . These are developed in various degrees, generally fine-structured and frequently disrupted. Their low diagnostic value may be demonstrated in some species, e.g. M. multicostatus . Some specimens have interstices without any trace of secondary costae and the other from the same population with quite well developed reticulate costae. Similarly, the shape of pronotum varies within some species. Eliminating the situation of abnormally developed pronotum, we can still find, that some specimens have wider pronotum than others within one species and also the degree of convexity of pronotal margins is frequently variable.
The shape of male genitalia and antennae usually differ between species and does not show substantial intraspecific variability. Some species share similar general appearance of the aedeagus, but differences can be found in the variously modified apical part of the median lobe. Morphology of male genitalia and the shape of the antennomere 3 are the primary sources of diagnostic characters at species level and these are the only characters enabling reliable species identification. However, even these characters may vary in some species: the lamella of antennomere 3 is developed in variable extent in M. bowringi and M. bocakorum . As for the genitalia, ventral-cavity varies in shape from oval to narrow within some species, and when the ventral cavity is small and oval, its position may also be variable.
The coloration patterns are rarely conspicuously variable within species. As the colouration of net-winged beetles is signaling their unpalatability (Bocak & Bocakova 2008) the complexes of syntopically occurring species usually share the same colour pattern and due to the high degree of endemism at the species level, seldom the range of one species simultaneously covers the area where different colour patterns evolved ( Bocak & Yagi 2010). We found two colour patterns only in M. dotatus : the specimen from Laos were uniformly orange, and those from Vietnam uniformly dark red. Another similar case is M. oreophilus reported by Kazantsev (2002). The dry stored specimens are darker than freshly collected material and may partly lose their original coloration.
Biology and Ecology. Adults, similarly to other net-winged beetles, prefer moist and shaded places with rotten trunks or logs lying in a close contact with soil, especially with shrubs along streams under the canopy of trees. They are mostly active in the morning, flying slowly at short distances, frequently found on the surface of leaves of lower shrubs or on the shaded areas of trunks. Macrolycus are not attracted to the light. In Laos and continental China, the adults are active in nature from April to early July corresponding to different latitudes and altitude. Larvae of the Chinese or Laotian species are unknown and we have only information on Japanese species ( Bocak & Matsuda 2003, Levkanicova & Bocak 2009). The larvae of Japanese Macrolycus live in decaying logs, under bark or in soft red-rotten wood.
Distribution. Similarly with most net-winged beetles, Macrolycus species occur in very restricted areas (Bocak 2000, 2007). We may roughly identify six areas of endemism ( Fig. 1 View FIGURE 1 ): (1) Russian Far East, Northeastern China and Korea with six Macrolycus . The region is characterized by continental climate and cold winters, the ecosystems are mostly lower elevation deciduous or conifer forests; (2) Japan incl. Ryukyu Islands encompassing a wide variety of ecosystems from the temperate zone of Hokkaido to the wet subtropical ecosystems of Iriomote and Ishigaki Islands in the South (16 species); (3) Taiwan with 6 species; (4) The high mountain regions of Yunnan, Sichuan, Gansu and Shaanxi provinces with deciduous and conifer forests (12 species); (5) Southeastern and central China with evergreen forests in lower elevations and deciduous forests in the highest altitudes, generally an area with prevalence of lower mountain ecosystems (7 species); The Himalayas and northernmost part of Thailand and Indochina with monsoon evergreen ecosystems (11 species). We have seldom found species distributed across two or more above defined regions except M. dominator known from Japan and Taiwan, and a few species occurring in the Far East and Japan (the record of M. flabellatus from Taiwan ( Kleine 1926) seems to be based on misidentification and we have not seen any specimen of M. flabellatus from this island. Although further research will surely expand ranges of some species, the geographic origin may provide some guidance for the identification of species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Macrolycus Waterhouse, 1878
| Li, Yun, Bocak, Ladislav & Pang, Hong 2012 |
Macrolycus
| Waterhouse 1878: 96 |