Philotheca freyciana, Rozefelds
|
publication ID |
https://doi.org/10.1071/SB22003 |
|
DOI |
https://doi.org/10.5281/zenodo.10974404 |
|
persistent identifier |
https://treatment.plazi.org/id/03F787B6-BD4E-FFB0-FF4A-0244FA6D846C |
|
treatment provided by |
Felipe |
|
scientific name |
Philotheca freyciana |
| status |
|
The status of Philotheca freyciana View in CoL warrants further investigation
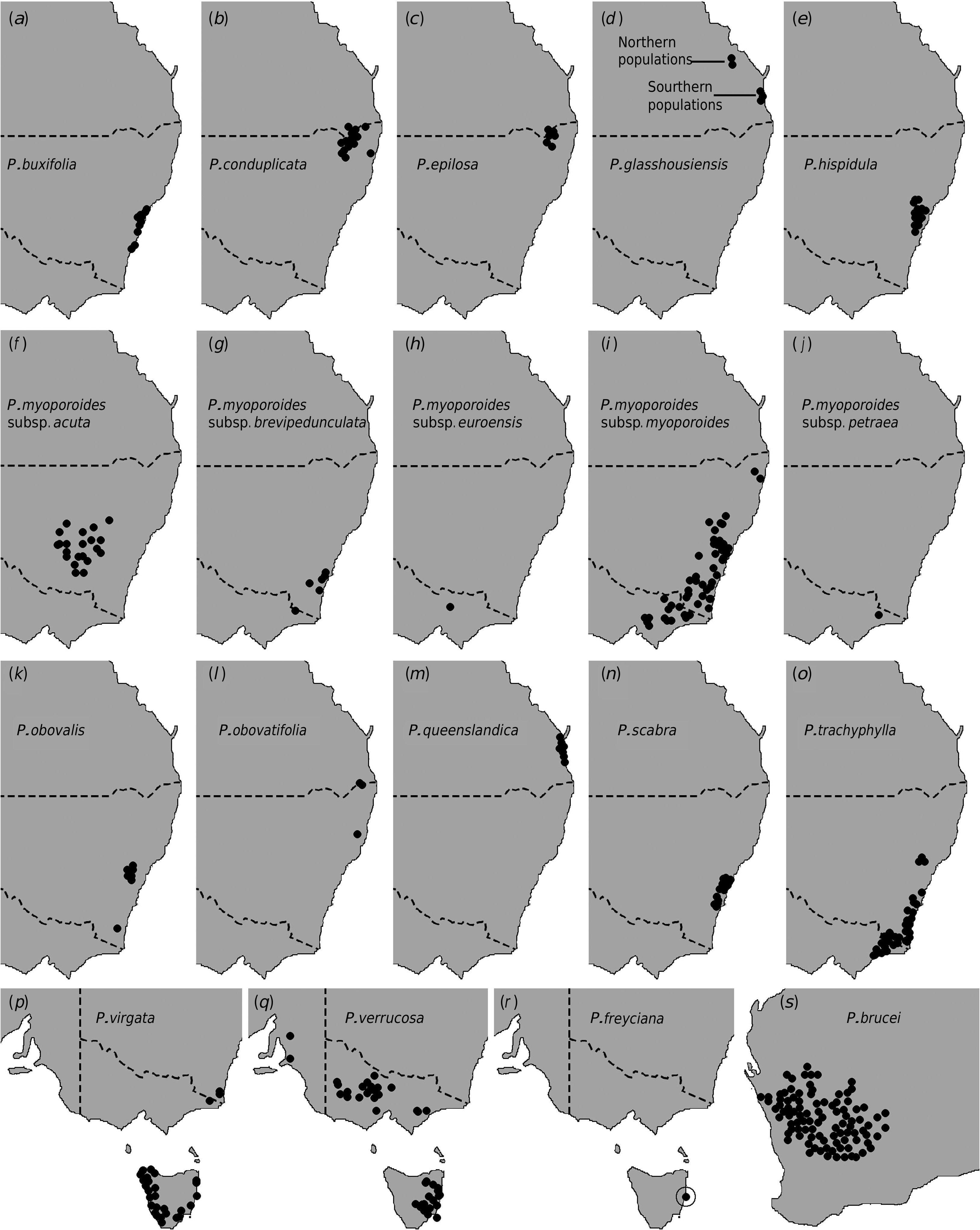
Philotheca freyciana View in CoL was described by Rozefelds (2001 a) for populations from Freycinet Peninsula, Tasmania (Tas.), that were previously included in the more widespread species P. verrucosa View in CoL (e.g. Wilson 1970; as Eriostemon verrucosus A.Rich. View in CoL ). Rozefelds (2001 a) distinguished P. freyciana View in CoL from P. verrucosa View in CoL on the basis of habit, leaf size and anther apex shape. Because of its limited distribution and small population sizes, P. freyciana View in CoL is listed as Endangered under both Australia’s Environment Protection and Biodiversity Conservation Act 1999 and Tasmania’s Threatened Species Protection Act 1995.
Our analysis placed the single sample of P. freyciana View in CoL in a clade with weak–moderate support ( PP 0.90, BS 74%) with samples of P. verrucosa View in CoL from Vic. and Tas. There is low sequence variation among samples in this clade and the BI consensus tree suggests that P. freyciana View in CoL could be nested within P. verrucosa View in CoL , although with little support ( PP 74, BS <50%). Given this result, and that morphological differences between the two species are slight ( Duretto 2009), their relationships and genetic distinctiveness are worthy of further investigation. This seems especially worthwhile given the conservation listings of P. freyciana View in CoL and the potential conservation funding that might be spent to preserve it. Such a study, using additional samples and genetic markers, is currently underway ( W. Neal in prep.).
Implementation of taxonomic changes
Although our results highlight the need for several taxonomic changes in sect. Erionema, these changes are not formally implemented here. One reason for this is that further morphological study is needed to assist with the delimitation of some taxa that are identified here on genetic grounds; this particularly applies to the two genetic groups within P. myoporoides subsp. myoporoides and to recognising the northern populations of P. glasshousiensis View in CoL as a distinct species. The other reason is that the generic circumscription of Philotheca is uncertain and we would prefer not to create new names or combinations for taxa until that is resolved. Our unpublished data, and previous studies ( Bayly et al. 2013), strongly suggest that Philotheca is not monophyletic but relationships of the four sections of Philotheca to each other and to related genera are yet to be clarified. It is possible that sect. Erionema might be recognised as a genus distinct from Philotheca . Some of us ( MJB and collaborators) are working to resolve relationships of this group and to propose a revised generic classification. Our intention is that new names or combinations for members of sect. Erionema (e.g. raising the segregate subspecies of P. myoporoides to species rank) would be published as part of that work, rather than creating additional, potentially briefly used names in the interim.
Implications for biogeography
The relationships resolved here in sect. Erionema indicate some interesting biogeographic patterns. These include: (1) the disjunction between western and eastern Australia (between Philotheca brucei and all other taxa); (2) the presence of potential deep geographical overlaps of two eastern clades (i.e. the pedunculate clade and the P. obovalis + P. trachyphylla + P. virgata clade); (3) early and substantial divergence of a south-eastern Qld lineage ( P. glasshousiensis + P. queenslandica ) within the pedunculate clade; (4) two distinct connections between the mainland and Tas. (i.e. P. verrucosa and P. virgata , both in separate clades in the phylogeny); and (5) striking geographic disjunction between the sister taxa P. myoporoides subsp. petraea and P. epilosa (~ 900 km; Fig. 1 c, j View Fig ), and between the northern populations of P. glasshousiensis s.l. and P. myoporoides subsp. acuta (~ 850 km; Fig. 1 d, f View Fig ).
The seeds from members of sect. Erionema usually fall within a few metres of the parent and, unlike those from other sections of Philotheca , have no distinctive features to promote dispersal by animals ( Armstrong 1991; Bayly 2001). It thus seems likely that long-distance dispersal has not been of major importance in the history of the group and that biogeographic patterns have mostly been shaped by vegetation shifts associated with past climatic and geological changes and a history of differentiation of allopatric taxa through vicariance.
The ages of divergences within sect. Erionema have not been estimated via molecular dating but some inferences can be made from previous work. We have not attempted molecular divergence dating here because fossils most suitable for calibration sit outside the family Rutaceae ( Pfeil and Crisp 2008; Bayly et al. 2013) in taxa to which alignment of ITS and ETS markers is problematic and therefore likely to lead to spurious results. Nonetheless, the previous dating study of Bayly et al. (2013), based on chloroplast markers that display a more conservative rate of change ( rcb L and atp B), did include one member of sect. Erionema ( P. buxifolia ) and representatives of the other three subgenera of Philotheca (including two samples of subg. Philotheca ). The genus was not resolved as monophyletic in that study and relationships within the group were poorly supported, which, apart from other uncertainties regarding calibrations and mutation rate estimation, clouds understanding of divergence times. The branch connecting P. buxifolia to other taxa in the tree, i.e. a possible stem age for sect. Erionema, was 18–34 Ma (mean 28 Ma). The crown age for the section could be much younger than this, but this estimate at least allows the possibility that sect. Erionema dates to the Paleogene. A Paleogene age would be consistent with a vicariant separation of western and eastern Australia, potentially in the mid Miocene, as inferred for a range of other plant groups of the temperate mesic zone ( Crisp and Cook 2007). A history on that timescale could also help to account for the presence of highly disjunct, potentially relictual lineages in south-eastern Australia, and the presence of multiple, deeply diverged but geographically overlapping clades.
The suggested relationship between P. myoporoides subsp. petraea and P. epilosa is particularly remarkable, given not just the distance between them, but also the presence of other members of the section in intervening areas. Despite the substantial distance, a relationship between the two taxa is morphologically plausible as they show a strong resemblance in leaf shape and size, as also noted by I. C. Clarke in annotations on one of the only two herbarium specimens of subsp. petraea ( MEL 2030756 A). However, if this resemblance is taken as evidence of relationship, a high level of conservation in leaf shape is implied despite substantial isolation in distance and presumably time, and across differing climates.
| BI |
Istituto Ortobotanico |
| W |
Naturhistorisches Museum Wien |
| L |
Nationaal Herbarium Nederland, Leiden University branch |
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
| A |
Harvard University - Arnold Arboretum |
| I |
"Alexandru Ioan Cuza" University |
| C |
University of Copenhagen |
| MEL |
Museo Entomologico de Leon |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |