Pereute charops (Boisduval, 1836)
|
publication ID |
https://doi.org/10.1080/00222931003633227 |
|
persistent identifier |
https://treatment.plazi.org/id/03F66F7D-AA20-BC3F-FE00-FC44FF18FCF7 |
|
treatment provided by |
Felipe |
|
scientific name |
Pereute charops (Boisduval, 1836) |
| status |
|
Pereute charops (Boisduval, 1836) View in CoL
This species occurs from Mexico to Venezuela and Peru, and comprises about seven subspecies (Lamas 2004). Two subspecies, including the nominate subspecies, are endemic to Mexico, while a third, P. charops nigricans Joicey and Talbot, 1928 , is restricted to Central America, occurring from southern Mexico through Guatemala to Nicaragua. The population further south from Costa Rica and Panama on which our observations of the life history were made, currently belongs to an undescribed subspecies endemic to Central America (Lamas 2004). In Costa Rica, P. charops occurs in cool montane areas from 1200 m to 2200 m on the Pacific slope ( DeVries 1987) where it is locally common in Cordillera de Tilarán (W. Haber, personal communication 2001) and Cordillera Central. It has occasionally been recorded at lower elevations, down to about 800 m in the San José Province ( Austin 1992), although the species possibly does not breed at these altitudes.
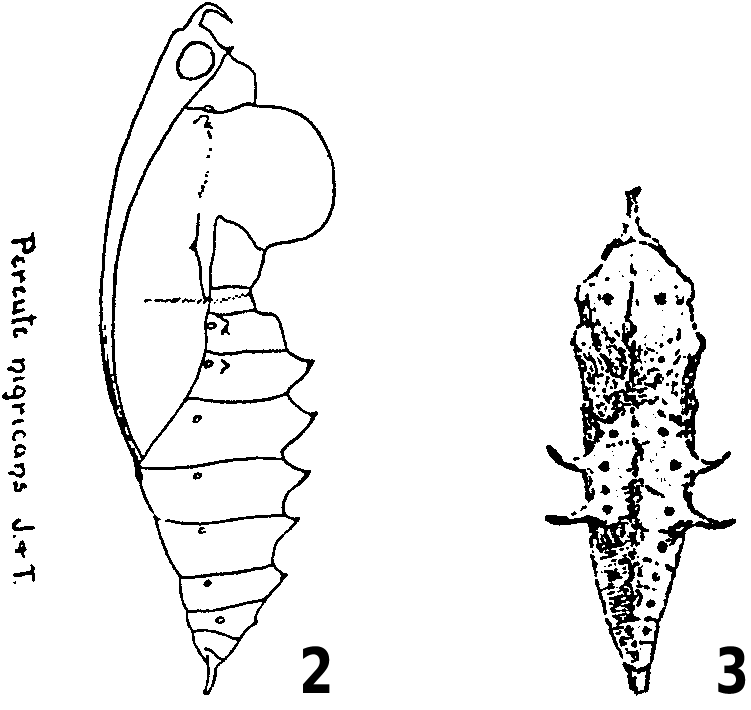
The larval food plants of P. charops are poorly documented in the literature. DeVries (1986, 1987) and Salazar (2004b) made only general reference to mistletoes (“ Loranthaceae ”) in Costa Rica and Mexico, respectively, while Guagliumi (1967), Alvarez and Alvarez (1984) and Briceño (1988) recorded Struthanthus dichotrianthus (listed as “guatepajarito” or “tiña”) as the larval food plant for Venezuela. Klots (1933) crudely illustrated the pupa of P. charops nigricans from Mexico ( Figure 2 View Figures 2–3 ), and Briceño (1988) depicted aggregations of final-instar larvae of P. charops venezuelana (Hopffer, 1878) from Venezuela. Alvarez and Alvarez (1984: 109) provided the following notes on the larvae and pupae of P. charops venezuelana :
Las larvas son de color marrón rojizo o color cacao y al termino de su desarrollo alcanzan de 3.5 centímetros de longitud. El cuerpo está cubierto de pelos o cerdas de color amarillo limón, de unos 2 mm de largo en los lados. En el dorso son más pequeños y menos numerosos. Poseen numerosos puntos amarillo limón por todo el cuerpo, concentrándose en el metatórax y primer segmento abdominal, hasta formar una tenue franja transversal. La cabeza es ovalada y del mismo color que el cuerpo, con algunas cerdas cortas. La región ventral es de un color marrón terroso. Son gregarias y crisalidan en grupas cabeza arriba... La crisálida es de color marrón y de unos 3 centímetros de longitud. En la región cefálica, en la frente, se observa una protuberancia cónica, con un gancho en forma de hélice, y también en la misma región se observan dos manchas globosas que corresponden a los ojos del insecto adulto. En la región media presenta una cresta que corresponde al tórax. Se observa una depresión que corresponde a la división entre el tórax y el abdomen y a continuación una cresta espinosa que corresponde al abdomen. Cada espina está formada por la unión de las dos partes de un segmento. La porción terminal del abdomen es puntiaguda. La crisálida está sujeta por una fina y resistente fibra de seda que pas por la depresión entre el tórax y el abdomen y también está sujeta por la porción terminal y puntiaguda del abdomen a la corteza del árbol. Estas crisálidas tienen bastante movilidad.
[The larvae are reddish-brown or chocolate and at the end of their development they are 3.5–4.0 cm in length. The body is covered by lemon-yellow hairs or setae of about 2 mm long on the side of the body and those of the dorsum are smaller and less numerous. They have numerous lemon-yellow spots all on the body, more concentrated on the metathorax and first abdominal segment, until they form a slight transverse fringe. The head is oval and of the same colour as the body with some short setae. The ventral region is brown. The larvae are gregarious and pupate in groups with head up... The chrysalid is brown and about 3 cm in length. On the cephalic region, on the frons, there is a conical protuberance, with a hook like propeller, and also in the same region there are two globose spots that correspond to the eyes of the adult insect. The medial region presents a crest that corresponds to the thorax. There is a depression that corresponds to the division between the thorax and abdomen. Each spine is formed by the union of the two parts of a segment. The terminal part of the abdomen is pointed. The chrysalid is fixed by a fine and resistant fibre of silk that passes through the depression between thorax and abdomen, and it is also fixed to the bark of the tree by the terminal pointed portion of the abdomen. The chrysalids are quite mobile.] (Our translation)
Briceño (1988) provided similar descriptions of the immature stages and included notes on the biology of the subspecies in Venezuela. Briceño (1988 p. 33) described the eggs as:
Los mismos son alargados en forma de barril con el ápice agudo. Presentan carinas o quillas longituinales. El corión es transparente por lo que el color amarillo del huevo es debido al contenido embrionario. Presenta color amarillo brilliante. El largo promedio observado fue de 1.2 mm y diámetro promedio fue de 0.50 mm.
[elongated and barrel shaped with an acute apex. They have longitudinal ribs. The chorion is transparent and the yellow colour of the egg is due to the embryo content. The colour is bright yellow. The average length was 1.2 mm and the average diameter was 0.5 mm.] (Our translation)
Briceño (1988) noted that the eggs are laid in clusters on the leaves of the larval food plant, ranging from 10 to 60 eggs per cluster, the larvae feed nocturnally, sheltering gregariously on the trunk of the host tree close to ground during the day, and that silken trails were laid down on the host tree to aid in mobility between the mistletoe and diurnal sheltering sites. Briceño (1988) also noted that larvae and pupae from several cohorts may aggregate together, with up to 800 individuals recorded in a single cluster (presumably comprising multiple cohorts), and that such numbers may completely defoliate the larval food plants, which take many months to regenerate. In Costa Rica, DeVries (1986 p. 304) remarked that he had observed “females oviposit large clusters of eggs” and “large groups of larvae feeding on leaves of the same epiphytic parasite. These larvae descended the tree trunk and pupated on the tree trunk”. Subsequently, DeVries (1987 p. 90–91) noted that the eggs are yellow and that “the pupae are black and dingy yellow, resembling a bird dropping (much like Catasticta )”. However, DeVries’ description of the pupa does not agree with our observations and most likely refers to a different taxon.
The following observations on the life history of P. charops were based on material reared from Costa Rica, mainly in the Central Valley, Cartago Province, at altitudes between 1150 m and 1450 m. In addition, we examined specimens of the larva and pupa from Mexico preserved in the BMNH and MCZ, respectively. In the BMNH, there are two well-preserved dried larval skins collected sometime around the turn of the twentieth century (P. Ackery, personal communication 2001). The specimens each have five labels, as follows: “BMNH DES No. Rh. 2870 Pereute charops charops Roths. coll.”, “Misantla, Veracruz (E. Gugelmann)”, “Misantla I No. 117”, “ Pereute charops [on Anona ]”, “Rothschild Bequest B.M. 1939-1”. The second larval specimen is similarly labelled except with “Rh. 2871” on the first label and only “ Pereute charops ” on the fourth label. In the MCZ, there are two historic intact pupae, each with two labels as follows: “Jalapa, Mexico ”, “ Pereute charops . Bdv”. In both specimens, the first label is typed while the second label is hand written. The age of the labels suggest the specimens were collected around the turn of the twentieth century (P. Ward, personal communication 2001), and almost certainly represent the specimens on which Klots (1933) based his illustration of the pupa of P. charops nigricans ( Figure 2 View Figures 2–3 ).
Immature stages
Egg
See Figures 64–67 View Figures 64–80 ; 1.1 mm high, 0.65 mm wide; bright yellow; bottle-shaped, with base flattened; chorion with numerous (approx. 30) fine longitudinal ribs, and a series of finer transverse lines between longitudinal ribs; apical rim with 11 prominent paler nodules.
First-instar larva
See Figures 68, 69 View Figures 64–80 ; 4.5 View Figures 4–13 mm long; head black, with several primary setae; body yellow, with numerous long, fine primary setae; setae colourless after eclosion, but most change to white after consuming food, except paired dorsal setae which change to dark brown in apical half; prothorax with a brown subdorsal plate bearing three setae (one twice length of other two), and three lateral setae; meso- and metathorax each with four setae (one subdorsal, two dorsolateral, one lateral), all in a transverse row; abdominal segments 1–9 each with five setae (one subdorsal, two dorsolateral, two lateral); abdominal segment 10 with a reddish-brown dorsal plate bearing eight setae.
Second-instar larva
See Figures 70–72 View Figures 64–80 ; 8 View Figures 4–13 mm long, head capsule 1.0 mm wide (n = 4); similar to second instar, but with numerous short dark brown secondary setae; prothorax with a broad, rectangular-shaped black dorsal plate, abdominal segment 10 with a black dorsal plate.
Third-instar larva
See Figures 73, 74 View Figures 64–80 ; 12 View Figures 4–13 mm long; similar to final instar.
Fourth-instar larva
See Figures 75, 76 View Figures 64–80 ; 24 View Figures 24–33 mm long; similar to final instar.
Fifth-instar larva
See Figures 77–85 View Figures 64–80 View Figures 81–91 ; 39 View Figures 34–44 mm long, head capsule 3.5 mm wide (n = 7); head black, with long yellow setae; body maroon to chocolate-brown, with numerous small yellow panicula from which arise long yellow setae (up to 3.5 mm long), and numerous very short brown secondary setae; prothorax with a broad, rectangular-shaped black dorsal plate bearing numerous long yellow setae; posterior end of metathorax with a double transverse row of long yellow setae; anterior end of abdominal segment 1 with a conspicuous yellow subdorsal plate bearing numerous long setae (approx. 24); abdominal segment 10 with a black dorsal plate bearing a few short setae; spiracles black.
Pupa
See Figures 86–88 View Figures 81–91 , 220, 221 View Figures 218–235 ; 26–28 View Figures 24–33 mm long, 7 mm wide (n = 22); dark shiny brown or dark reddish-black, becoming lighter orange-brown with black in intersegmental areas on abdomen; head with eye black, a short prominent anterior projection, and a small subdorsal protuberance posteriorly; anterior projection stout, upturned and bifurcated apically and T-shaped; prothorax with two black subdorsal plates, and a pronounced longitudinal dorsal ridge; mesothorax with a pronounced longitudinal dorsal ridge, a double rounded lateral protuberance at base of fore wing, and a broad lateral ridge posterior to lateral protuberance; metathorax with a shallow longitudinal dorsal ridge; abdominal segment 1 with a very small dorsolateral protuberance; abdominal segments 2 and 3 each with a dorsolateral protuberance; abdominal segment 4 with a weak dorsolateral protuberance; abdominal segment 2 with a middorsal ridge; abdominal segments 3–8 each with a prominent middorsal projection, terminating in a tooth-like point at apex; abdominal segment 9 with a small subdorsal protuberance; spiracles black.
After the prepupal stage, the head and wing cases were initially dark greenishgrey and the dorsal region of the thorax and abdomen were initially dull reddishbrown with a few yellow flecks; however, after a few hours the colour of the pupa darkened to shiny dark brown or dark reddish-black.
Larval food plants
In Costa Rica, the immature stages were commonly found associated with several mistletoes in the families Loranthaceae and Viscaceae parasitizing various host trees ( Figures 28, 29 View Figures 24–33 ). However, only three mistletoes were considered to be likely food plants, namely: Struthanthus sp. aff. orbicularis, Phoradendron undulatum (Pohl ex DC.) Eichler and P. tonduzii Trel. , with many records for the two Viscaceae species ( Appendix 1). Only for P. undulatum ( Figures 30 View Figures 24–33 , 83 View Figures 81–91 ) were the immature stages (eggs, early-instar larvae) actually found on the foliage, and in captivity the larvae readily consumed the leaves of this mistletoe. The two other species are listed based on unequivocal association, that is, the mistletoes were the only species growing on the host tree on which the immature stages (late-instar larvae, pupae, pupal exuviae) were found. Antidaphne viscoidea Poepp. and Endl. frequently grew together with Phoradendron undulatum on the same host tree, Psidium guajava L. ( Myrtaceae ), but we consider A. viscoidea an unlikely food plant because the immature stages were never found on the foliage of this species and, where two species co-occurred, larvae and/or larval feeding damage were only observed on Phoradendron undulatum .
Biology
Eggs were laid in loose batches on the upper- or underside of new leaves of the larval food plant. We recorded two cohorts of eggs comprising 110 and 149 individuals, but a cohort of newly emerged first-instar larvae collected from Río Macho in Orosi Valley totalled 266 individuals ( Figure 68 View Figures 64–80 ). In addition, we recorded several cohorts comprising mid-instar larvae, late-instar larvae or pupae that exceeded 100 individuals. These observations indicate that females are capable of laying exceedingly large clutches of eggs, and accord with observations made by DeVries (1986) who noted that females lay large clusters of eggs on the food plant. Prior to hatching, the apical portion of the egg changed to brown owing to development of the larval head capsule. The eggs hatched synchronously, over a period of 30 h. On hatching, the firstinstar larva ate a small hole just below the apex of the chorion through which it escaped; once free it then proceeded to devour part of, but usually not the entire, chorion. However, some newly hatched larvae were observed feeding on egg shells other than their own, as well as fertile eggs in which the developing larvae had yet to emerge. A few first-instar larvae were also observed feeding on the bodies of their siblings, leaving behind only the head capsule and some degenerated body tissue. From a cohort of 149 eggs, about 20 (13%) larvae were lost to such acts of cannibalism. The larvae fed gregariously and spun considerable quantities of silk over a leaf before eating it, particularly in the late instars. First-instar larvae usually “skeletonized” the leaf by grazing the surface, whereas subsequent instars consumed the entire leaf. When disturbed or molested, they reared their heads up and arched the anterior part of their body backwards to exude a green fluid from the mouth. Observations made from a sample of cohorts (n = 10) representing all larval instars, together with our captive rearing, indicated that during the early instars (I, II) the larvae remained on the foliage of the larval food plant, whereas the late instars (IV, V) sought shelter sites on the host tree during the day where they sat motionless, returning to the mistletoe clump to feed at night. The change in foraging and resting behaviour apparently occurred during instar III. The diurnal resting sites of late-instar larvae were located on the lower trunk of the host tree ( Figures 28, 29 View Figures 24–33 ), ranging from the base to 3 m from ground level, but generally below 1 m from the ground. The larvae formed a tight compact cluster ( Figure 82 View Figures 81–91 ) and, on trees in which the trunk was not vertically straight (e.g. the trunk of Psidium guajava frequently grows at an angle), they were usually located on the lower side of the trunk where they were sheltered and more protected from rain and possibly predators/parasitoids. On a few occasions, the shelter sites comprised a hollowed section of the trunk. The distance between the diurnal sheltering site and the nearest mistletoe clump varied from 1.5 m to 20 m, but was frequently more than 8 m. The late-instar larvae usually dispersed from the diurnal sheltering site around sunset, ascending in single file or procession along a conspicuous silken trail laid down on the trunk and branches of the host tree to reach the larval food plant ( Figure 81 View Figures 81–91 ). Once they reached the food plant, the larvae dispersed in small groups to feed on the leaves throughout the night. Observations made at Río Macho suggested that larval dispersal from the diurnal aggregation site to the food plant was dependent on ambient light conditions rather than on time of day or angle of the sun. For example, on one occasion when conditions were very overcast with some drizzle, a cohort of instar IV larvae were noted to ascend the host tree and move onto the mistletoe clump at 10:30 h. Before ascending the host tree, each larva rotated its head left and right for about 20 min. Pupae were found in the same situations as the larval diurnal shelter sites. They were attached head upwards to a dense layer of silk, which had been spun by the larvae on the trunk of the host tree, by the cremaster and a central girdle. When disturbed or touched, the abdomen of the pupa twitched or wriggled vigorously. Such twitching invariably caused adjacent pupae to twitch, thereby setting off a chain reaction with the effect of causing the whole cohort to wriggle simultaneously. In captivity, when reared at room temperature (23ºC ± 1ºC), adults of a given cohort emerged simultaneously; following emergence they quickly expanded their wings but were not capable of flight for at least 12 h until their wings had dried and hardened. A mass emergence event of an exceptionally large cohort ( Figure 9 View Figures 4–13 ) was once observed at Monteverde in July 2001 (W. Haber, personal communication 2001). Following emergence, the adults remained suspended for 1–2 d from their empty pupal shells attached to the trunk of the host tree before they dispersed and took flight.
In the field, adults were observed flying mainly during the morning, from 08:00– 12:30 h. DeVries (1987) noted that adults fly in the canopy with a characteristic and conspicuous slow, flutter-sailing flight; the males establish territories by patrolling along forest edges or in light gaps with long, circling flights during the morning until midday, and they defend these areas vigorously against rival males. W. Haber (personal communication 2001) has also noted that the males establish territories, from about mid-morning to midday, by patrolling around the crowns of the tallest trees growing in the rainforest or along the forest edge. Both sexes readily feed from flowers, including Duranta (Verbenaceae) (W. Haber, personal communication 2009), Poinsettia (Euphorbiaceae) (T. Carman, personal communication 2000), Clibadium surinamense L. ( Asteraceae ) ( Vega and Gloor 2001), Fuchsia paniculata Lindl. (Onagraceae) , Eupatorium and other Asteraceae ( DeVries 1987) . Males were also observed drinking from moist sand/soil on dirt tracks, from 08:30–15:00 h.
The life cycle, from egg to adult, was completed in 9–10 weeks when reared at constant temperature (16–18°C) (egg ≥ 5 d; larva, 41–43 d [duration of larval instars as follows: I, 5 d; II, 5 d; III, 5–6 d; IV, 8 d; V, 18–19 d]; pre-pupa, 1–2 d; pupa, 17–18 d). The pupal duration was slightly shorter (14 d) when reared at 25°C. The final-instar larvae, after about 10 d of feeding, became quiescent for 8–9 d, during which time they remained huddled together and did not feed; they then entered the pre-pupal stage, which lasted 36–48 h, before pupating. In Costa Rica, P. charops probably breeds throughout the year. Adults have been recorded in each month, but they are very seasonal in abundance (J. Wolf, personal communication 2000; W. Haber, personal communication 2001), possibly owing to the synchrony of development of individual cohorts or fluctuation in numbers of parasitoids. DeVries (1987) remarked that the species is most abundant during the dry season, which extends from December to April at San José in Cordillera Central. Phoradendron undulatum , a common larval food plant, produces an almost continuous growth of leaves (W. Haber, personal communication 2001) and is thus available to early-instar larvae throughout the year.
A relatively high level of mortality among pupae owing to natural enemies and disease was evident. In a sample of 6 cohorts (ranging from 7– 80 pupae per cohort), the mortality rate varied from 68 to 100%, with an average of 82% ( Appendix 1). Pooling all samples for the six cohorts yielded the same overall death rate of 82% (n = 225 pupae). Parasitized pupae were generally darker brown-black in colour and characterized by the presence of a conspicuous hole in one of the wing-cases through which the parasitoid had escaped, although on one occasion a parasitoid was noted to emerge from the dorsal surface between abdominal segments 1 and 2 (R. Eastwood, personal communication 2001). The parasitoids included two species of Tachinidae (Diptera) : Jurinia and Trichophora ( Figure 84 View Figures 81–91 ) (M. Wood, personal communication 2002); and two species of Chalcididae (Hymenoptera) : Brachimeria and Conura (P.E. Hanson, personal communication 2002). These parasitoids were also observed to attack cohorts of final-instar larvae and pre-pupae; typically the parasitoid would land close to a cohort of larvae and then walk around its perimeter, attempting to oviposit on the bodies of individual larvae. In response to presence of parasitoids, the larvae frequently raised the head and anterior portion of the body (thorax) upwards in rapid succession, behaviour that appeared to deter further attack. The behavioural response of the larvae was reminiscent of that observed in larvae of sawflies ( Hymenoptera : Pyrginae), commonly known as “spitfires”, when provoked ( Carne 1962).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |