Catasticta hegemon Godman and Salvin, 1889
|
publication ID |
https://doi.org/10.1080/00222931003633227 |
|
persistent identifier |
https://treatment.plazi.org/id/03F66F7D-AA07-BC14-FE1F-FB52FB8EFD37 |
|
treatment provided by |
Felipe |
|
scientific name |
Catasticta hegemon Godman and Salvin, 1889 |
| status |
|
Catasticta hegemon Godman and Salvin, 1889 View in CoL
This species ( Figure 17 View Figures 14–23 ) ranges from Costa Rica to Ecuador and is divided into three subspecies (Lamas 2004). The nominate subspecies, C. hegemon hegemon Godman and Salvin, 1889 , on which our observations of the life history were made, is endemic to Costa Rica and Panama of Central America ( DeVries 1987; Lamas 2004). In Costa Rica, it occurs commonly in wet forest habitats from 1000 m to 2500 m ( DeVries 1987). The life history of C. hegemon has not previously been reported. The following observations on C. hegemon hegemon were made in Costa Rica at Monteverde, Puntarenas Province, on the Pacific slope in Cordillera de Tilarán at elevations between 1400 m and 1500 m, mainly in January 2000 and October 2001. Additional observations on adult behaviour were made at Parque Nacional Chirripó (1840 m a.s.l.), San José Province, on the Pacific slope in Cordillera de Talamanca in February 2009.
Immature stages
Egg
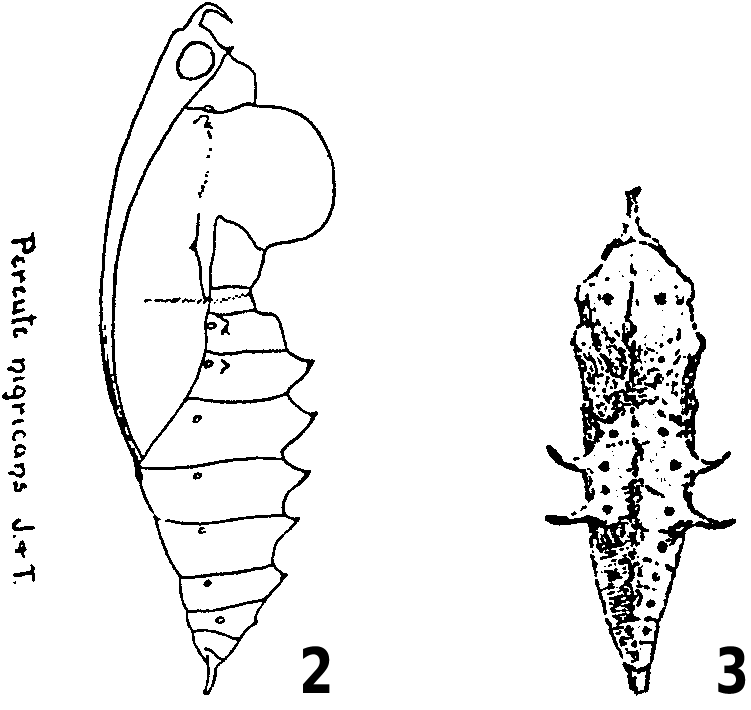
See Figures 162–164 View Figures 162–181 ; 0.8 mm high, 0.5 mm wide; white when newly laid, later changing to cream; barrel-shaped, with base flattened and much narrower in width than middle; chorion with numerous (approx. 28–30) longitudinal ribs, and a series of finer transverse lines between longitudinal ribs; apical rim with six to seven prominent paler nodules.
First-instar larva
See Figures 165, 166 View Figures 162–181 ; 3.5 View Figures 2–3 mm long, head capsule 0.4 mm wide (n = 8); head orangebrown, with a few colourless primary setae; body pale lemon after eclosion, changing to green after consuming food, with numerous long, fine colourless primary setae; paired black dorsal setae, bifurcated at apex, on meso- and metathorax and abdominal segments 1–8; prothorax with dorsal plate bearing six setae (in two groups, three on either side of middorsal line), two dorsolateral setae and a lateral seta; meso- and metathorax each with four setae (one subdorsal, two dorsolateral, one lateral), all in a transverse row; abdominal segments 1–9 each with four setae (one subdorsal, two dorsolateral, one lateral); abdominal segment 10 with dorsal plate bearing setae.
Second-instar larva
See Figures 167, 168 View Figures 162–181 ; 5 View Figures 4–13 mm long; similar to first instar, but head darker brown, with prominent orange-brown band in ventrolateral region; body green with numerous cream and yellow panicula bearing colourless setae, and numerous short brown secondary setae; prothorax with dorsal plate centred black.
Third-instar larva
See Figure 169 View Figures 162–181 ; 8 View Figures 4–13 mm long, head capsule 1.1 mm wide (n = 20); similar to fourth instar.
Fourth-instar larva
See Figures 170, 171 View Figures 162–181 ; 14 View Figures 14–23 mm long, head capsule 1.8 mm wide (n = 17); similar to final instar, but head dark brown with prominent orange-brown band in ventrolateral region, and numerous dull orange panicula bearing setae; body with long white setae.
Fifth-instar larva
See Figures 172–177 View Figures 162–181 ; 27 View Figures 24–33 mm long, head capsule 2.8 mm wide (n = 5); head dark brown partly covered with pale blue protuberances which coalesce; body bright green, with darker a green middorsal line, numerous white and cream panicula from which arise white setae, and numerous shorter brown setae; prothorax with dorsal plate centred black and bearing setae; abdominal segment 10 with pale blue dorsal plate bearing setae.
Pupa
See Figures 178–181 View Figures 162–181 ; 18 View Figures 14–23 mm long (excluding anterior projection), 5 mm wide (n = 15); bright green, with a few scattered black spots; head with a prominent red anterior projection, and a smaller black subdorsal projection posteriorly; anterior projection long (4 mm), oriented upwards, and bifurcated at apex; prothorax with a pronounced red longitudinal dorsal ridge; mesothorax with a pronounced red longitudinal dorsal ridge, a double rounded lateral protuberance at base of fore wing, and a broad lateral ridge posterior to lateral protuberance; abdominal segments 2–4 each with a long, spine-like yellow dorsolateral projection tipped with black; abdominal segments 2–8 each with a spine-like red middorsal projection (especially long on segments 3–7); cremaster green or pale orange.
The immature stages are similar to those of C. sisamnus , C. flisa and C. ctemene , but in C. hegemon the final-instar larva is distinguished by the appearance of patches of aqua-blue on the head capsule and dorsal plate of abdominal segment 10. The pupa is most similar to C. ctemene in colour pattern, both of which have the anterior projection on the head coloured red, but the black spots are less well developed.
Larval food plants
In Costa Rica, eight cohorts of the immature stages were recorded on a single large clump of Antidaphne viscoidea parasitizing the tree Feuilleea punctata (Willd.) Kuntze (Fabaceae) growing in drier rainforest with a low canopy (<10 m high) ( Appendix 1). The mistletoe clump grew beneath canopy of the host tree about 5 m from ground level ( Figure 39 View Figures 34–44 ).
Biology
Eggs were laid in tight compact clusters, usually on the upperside of large thick “leathery” leaves at the apex of a branch or just below the most terminal leaf of a branch, ranging from 30 to 44 eggs per cohort (n = 6). The egg clusters were usually laid in circular masses 8–10 mm in diameter in the apical third of the leaf. The larvae hatched synchronously and the first instars proceeded to devour the chorion before consuming the foliage. In the early instars, they grazed the leaf surface giving the leaf a “skeletonized” appearance, but later they consumed whole sections of the leaf. All instars fed and moulted gregariously. When moulting, they resided on the underside of a leaf where they were well camouflaged, the green body closely matching that of the leaves while the brown head capsule resembled dead patches of leaf tissue. The petiole of the leaf on which larvae were feeding or moulting was strengthened with extensive amounts of silk. In captivity, the larvae pupated solitarily, secured to a pad of silk by the cremaster and a central silken girdle. Following emergence, adults were ready for flight within 3–4 h.
DeVries (1987) noted that adults are frequently seen flying in light gaps at or near the canopy. At Monteverde, males ( Figure 17 View Figures 14–23 ) were observed to exhibit territorial behaviour within the breeding area in the morning during sunny conditions. Each male would typically establish a territory in a small light gap, that is, a prominent opening in the dense forest canopy where light reached the understorey. Within the light gap, they perched on certain objects such as foliage, dead branches and other prominent features 1–3 m above ground level; regular short flights in the open space of the light gap were made, after which they returned to settle on the same object. Their flight was rapid and jerky, and the resident male was observed to chase conspecific males and other butterflies, including Catasticta (e.g. C. ctemene ), out of the territory. On several occasions, when two males were engaged in twirling aerial combat, an audible clicking sound of the wings was evident, similar to that recorded for C. flisa ( DeVries 1987) . When settled, the wings were usually closed or just slightly opened. However, they also basked by opening their wings at an angle of 180º or sometimes slightly depressed over the body (>200º) with the dorsal surface exposed towards the sun; however, after a while (or later in the day when conditions were warmer) dorsal basking was less frequent and the wings were held tightly closed over the body. At a breeding site in Monteverde, four light gaps each occupied by a single male were encountered over an area measuring several hectares. When a male was collected and removed from the light gap, another male was found to enter the territory and take up residency within 30 min. These observations suggest limited spatial availability and strong intraspecific competition for these landmarks, which presumably are used as encounter sites to locate receptive females. A female was observed ovipositing on the larval food plant, growing about 30 m from one of these light gaps occupied by a male, at 09:50 h on 11 October 2001. After laying the final egg she rested on a leaf just above the cohort for a few minutes before departing. Males were recorded feeding on flowers of Asteraceae and ornamental Duranta erecta L. ( Verbenaceae ).
The life cycle, from egg to adult, when reared at room temperature (c. 19–22°C) was completed in approximately six weeks (egg, 6 d; larva, 27–30 d [duration of instars as follows: I, 5 d; II, 4–5 d; III, 4–5 d; IV, 6 d; V, 8–9 d]; pupa, 8–10 d).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.