Protonemura shimizui Murányi & Gamboa, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4718.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:16DB86F7-38BF-4F6A-8A84-53A05789B539 |
|
persistent identifier |
https://treatment.plazi.org/id/03F2AF3C-FFC6-7D18-FF47-23C6B172F810 |
|
treatment provided by |
Plazi |
|
scientific name |
Protonemura shimizui Murányi & Gamboa |
| status |
sp. nov. |
Protonemura shimizui Murányi & Gamboa View in CoL , sp. n.
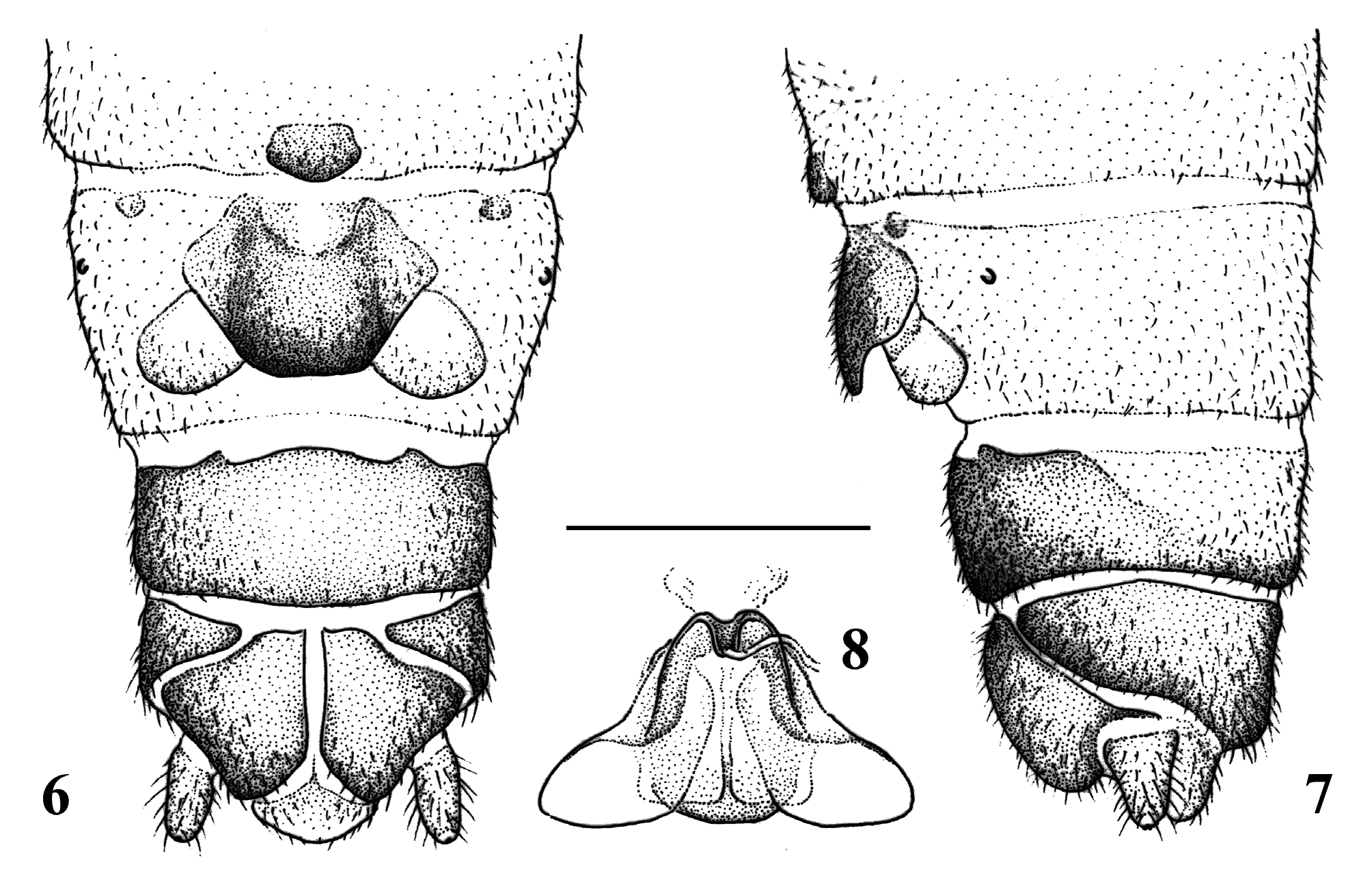
( Figs. 1–9 View FIGURES 1–5 View FIGURES 6–8 View FIGURE 9 , 11–12)
Protonemura View in CoL sp. n. — Gamboa et al. 2019: 8. (molecular analysis).
Type material: Holotype male: JAPAN: Ehime prefecture, Saijo City, forest seep and brook along Ishizuchi Skyline , E of Tuchigoya, 1450m, N33°45.322’ E133°09.436’, 15.x.2015 (coll.no.41), leg. Y. Kaetani, D. Murányi, S. Yamano ( LBM) GoogleMaps . Paratypes: same locality and date: 2♀ ( BYU) GoogleMaps , 17♀ ( HNHM) GoogleMaps , 2♀ ( LBM) ; same locality, 4.xi.2016 (coll.no.280), leg. Sz. Czigány, A. & D. Murányi: 1♀ ( HNHM) .
Diagnosis. Male: median lobe of the paraproct with very short inner expansion; outer lobe very large and black, apex with medial membranous field and acute ventral, and curved dorsal branch; epiproct with double apical filament, dorsomedial groove and short but strong ventral bulge. Female: pregenital plate very small; subgenital plate small, posterior edge not notched; vaginal lobes large.
Description. Medium sized species, macropterous. Forewing length: holotype male 8.2 mm, paratype females 8.8–10.5 mm. Head dark brown, antenna brown, palpi yellowish. Thorax brown, pronotum trapezoid with rounded corners, wider than long, rugosities indistinct. Cervical gills brownish and bear fine setation, without subterminal constriction, length equal to forecoxa. Legs yellowish but base and apex of femora and tibiae, as well as tarsi darker; wing membranes brownish, venation brown. Pilosity short and indistinct.
Male abdomen ( Figs. 1–5 View FIGURES 1–5 ): Sterna 1–8 membranous with paired small, anterolateral sclerites. Hypoproct round- ed, less than half wide than sternum 9 width, apical part long, gradually tapering and blunt; vesicle claviform, twice longer than wide. Paraproct inner lobe simple, much shorter than median lobe, apex blunt. Median lobe with wide, well sclerotized shield-like base, inner expansion (tigellum) very short and tooth-like, membranous apex enlarged and covered with setae, nearly as long as outer lobe. Outer lobe very large and black besides a medial membranous portion of the apex; basal half is erect then curved dorsad, apex with a straight and acute ventral branch that points apically, and a long dorsal branch that is curved inwards then outwards and bearing two strong apical spikes. Cerci simple, short and rounded, base weakly connected to the paraproct base; terminal wart inconspicuous. Terga 1–6 membranous with paired, elongated anterolateral sclerites. Terga 7–9 with entire antecosta; tergum 7 mostly sclerotized but lacks spinules, tergum 8 with small posteromedial membranous field and a few spines, tergum 9 with medial membranous field nearly as wide as third of segment width and bearing numerous spines. Tergum 10 with a rounded membranous area medially, beneath the apex of epiproct; a few short spikes surround the area, apical lobe wide. Epiproct strong and relatively wide, with distinct, double apical filament; in dorsal view, the apical half is widened and rounded; in lateral view, the highest is at the apical third. Dorsal sclerite forked after a short base, branches run laterally over midlength and ends with blunt apex, delimit a large, membranous dorsal area that is grooved medially. Ventral sclerite evenly narrow, connected with the basal sclerite; its ventral edge is distinctly bulging in the apical third, the short bulge is armed with relatively few and short spines; apex abruptly upturned and ends in the paired filament.
Female abdomen ( Figs. 6–8 View FIGURES 6–8 ): Terga 1–8 light brown to reddish, full membranous; tergum 9 darkly sclerotized in apical half, tergum 10 full sclerotized and dark; epiproct lightly sclerotized. Sterna 1–8 light brown to reddish, membranous with paired small, anterolateral sclerites usually lacking distal to sternum 3. Sternum 7 with small but distinct, rounded or triangular pregenital plate; width of the plate is less than one fifth of segment width. Subgenital plate small, its width is less than half of the segment width; posterior edge evenly rounded or only slightly undulate, not notched; colour of the plate brown to dark brown; rounded in lateral view, apical half slightly elevated over vaginal lobes. Vaginal lobes large, rounded and pale, stretching close to segment edges. Sterna 9 and 10 dark brown, simple. Paraprocts dark brown, apex rounded and not elongated; cerci simple. Inner genitalia: a wide plate origi- nates between the subgenital plate and vaginal lobes, covering the genital opening; the plate is weakly sclerotized on most portions but apical half with lateral edges folded and thickened, attached to basal part of vaginal lobes; apex of the plate joins to the spermathecal ductus with an upcurved, channel-like sclerotized projection.
Larva: Unknown.
Affinities. Due to the double apical filament and dorsomedial groove of the male epiproct, the new species shall be included in the orbiculata species group sensu Shimizu (1998), however, the vestigial expansion (tigellum) of the paraproct’s median lobe may suggests affinities towards the towadensis species group sensu Shimuzu (1998). The unique outer lobe of the paraproct easily distinguish the male from all congeners classified in both species groups, only P. angulata Shimizu, 1998 from northern Honshu have slightly similar structure but that species clearly differs in epiproctal structures and by the long expansion of the paraproct’s median lobe. The female of the new species is less distinctive but can be distinguished from all Japanese congeners by the combination of very small pregenital plate, small and posteriorly not notched subgenital plate, and large vaginal lobes.
Genetics ( Fig. 9 View FIGURE 9 ). Haplotypes formed a genetic cluster separated from the other Japanese Protonemoura species studied ( P. orbiculata Shimizu, 1998 ( orbiculata species group, MK132208 View Materials ); P. kohnoae Shimizu, 1998 (towadensis species group, MK132201 View Materials )), with 6% of nucleotide substitution rate. It is worth to mention that Protonemura sp. n. 1–4 in Gamboa et al. (2019) do not refers on new species but unassociated larvae.
Distribution and ecology. The species was only found at a single high elevation rheocrene of the Ishizuchi Range, despite sampling in several similar habitats at different elevations across the area. The type locality is a complex of a small forest brook with stony-gravely bed mixed with wood debris and silty patches, mossy seeps and hygropetric habitats (Figs. 11–12). The water has moderately fast flow, with cascading sections over hygropetric and mossy patches. The brook is in the upper forest zone with dense undergrowth.
The habitat was sampled four times, in October 2015, May 2016, August 2016 and November 2016. The new species was presented by many females and a single male in October, and by a single female in November, but no adults were found during spring and summer collecting and our attempts to find larva of the new species was unsuccessful. In May, adults of seven species were found ( Perlomyia kappa Sivec & Stark, 2012 , Indonemoura nohirae ( Okamoto, 1922) , Nemoura cercispinosa Kawai, 1960 , N. naraiensis Kawai, 1954 , unidentified females of a further Nemoura sp., and two unidentified species of Sweltsa ), among these taxa, P. kappa was the most abundant. No stoneflies were found in August. In October, the most abundant species was Amphinemura decemseta Okamoto, 1922 , then the new species, whereas adults of A. longispina Okamoto, 1922 , Indonemoura nohirae , N. cercispinosa and N. longicercia ( Okamoto, 1922) were found in low numbers. In November, the only stonefly caught was the single female of the new species. The only Nemouridae larvae found at the type locality belong to an unidentified Nemoura species, found in a hygropetric microhabitat.
Etymology. The new species is dedicated to Dr. Takao Shimizu, in recognition of his leading contributions to stonefly taxonomy, and in gratitude for his help during our Nemouridae and Capniidae studies. The name is used as the genitive of a noun of male gender.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Protonemura shimizui Murányi & Gamboa
| Murányi, Dávid, Gamboa, Maribet & Watanabe, Kozo 2020 |
Protonemura
| Gamboa, M. & Muranyi, D. & Kanmori, S. & Watanabe, K. 2019: 8 |