Scutellera nepalensis (Westwood, 1837)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5092.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:3CAF2F90-A654-40B7-BABA-A0035A1A6DE8 |
|
DOI |
https://doi.org/10.5281/zenodo.5879771 |
|
persistent identifier |
https://treatment.plazi.org/id/03F287DA-8117-AF3F-FF45-B56DFAF2FD80 |
|
treatment provided by |
Plazi |
|
scientific name |
Scutellera nepalensis (Westwood, 1837) |
| status |
|
Scutellera nepalensis (Westwood, 1837)
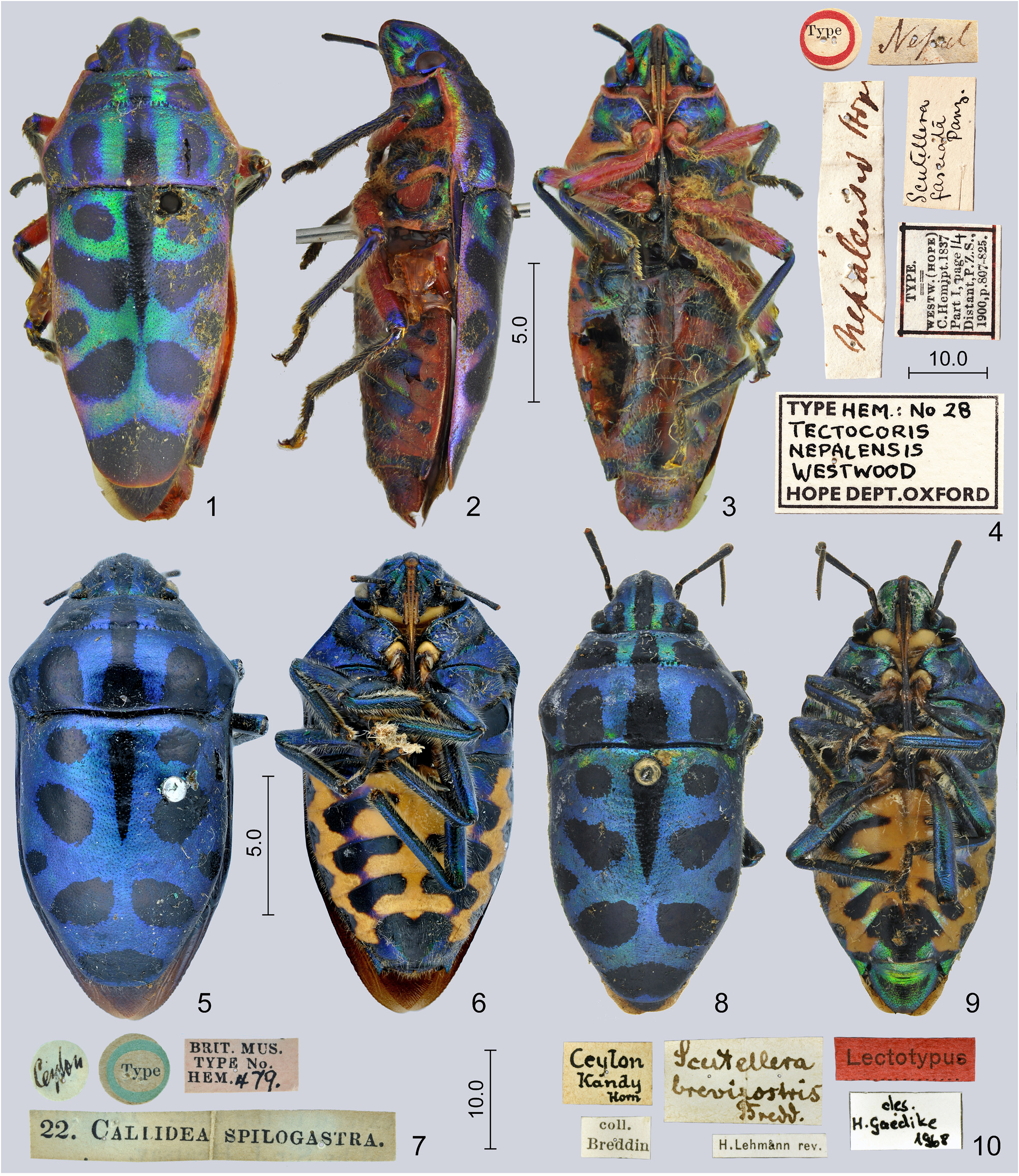
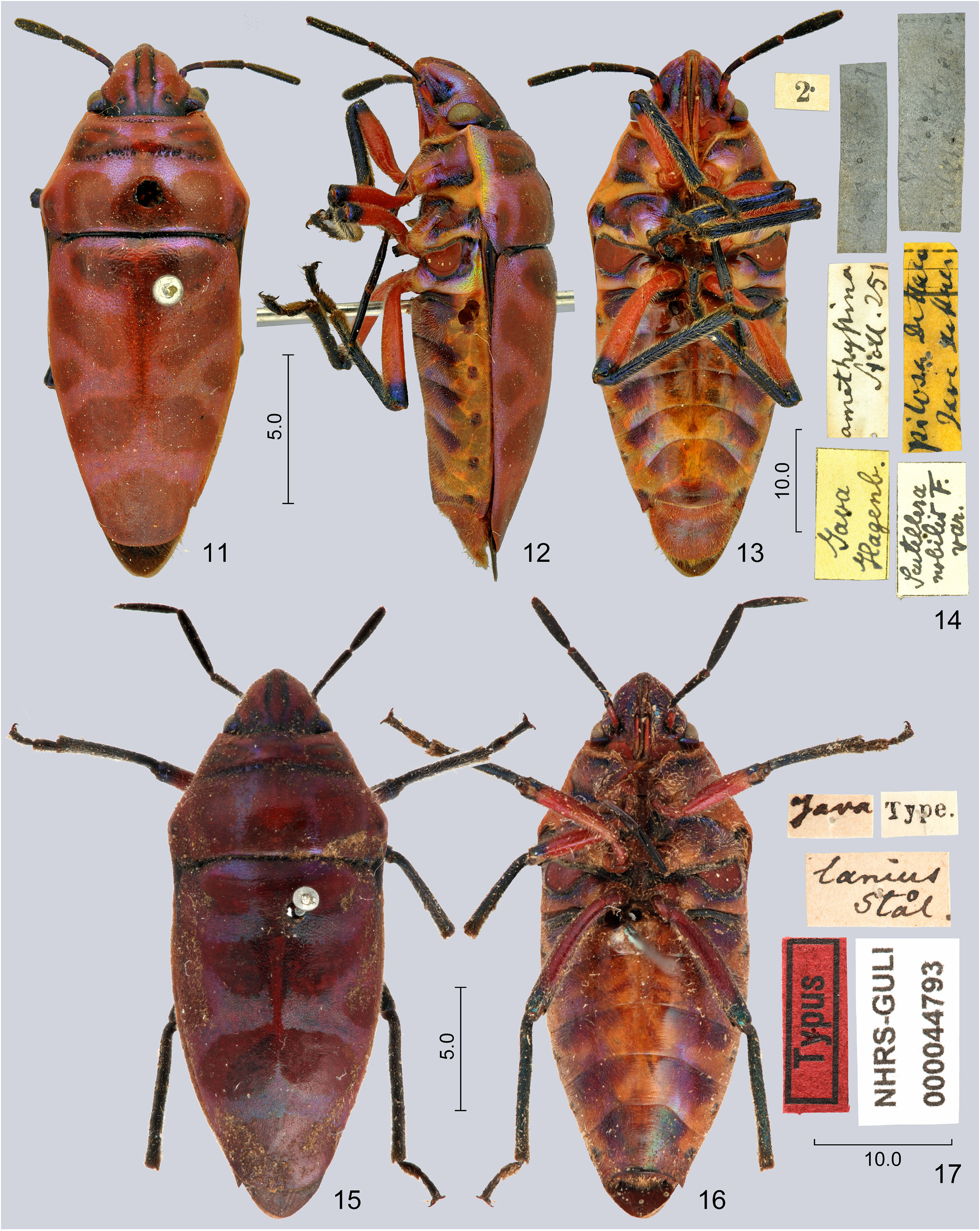
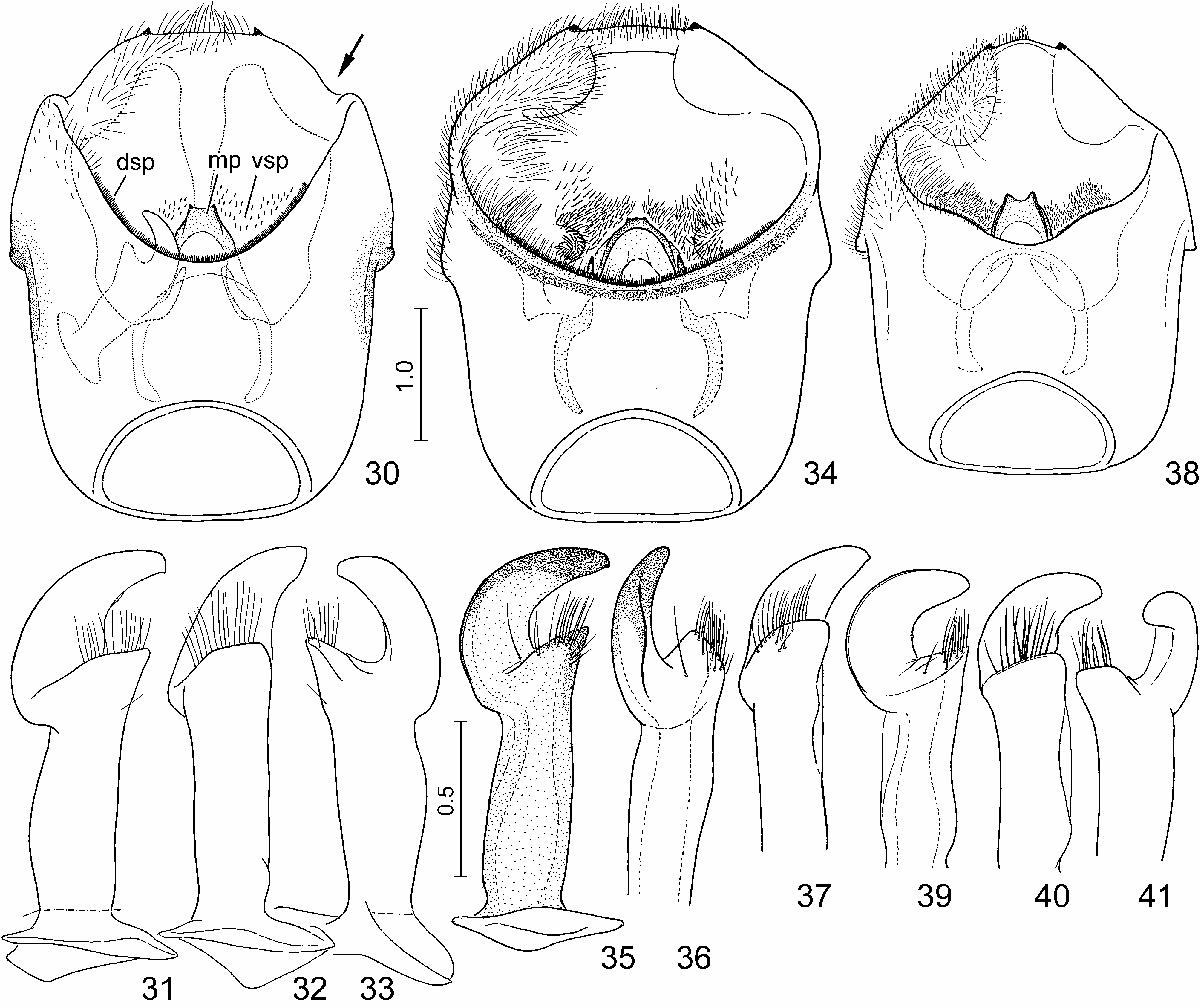
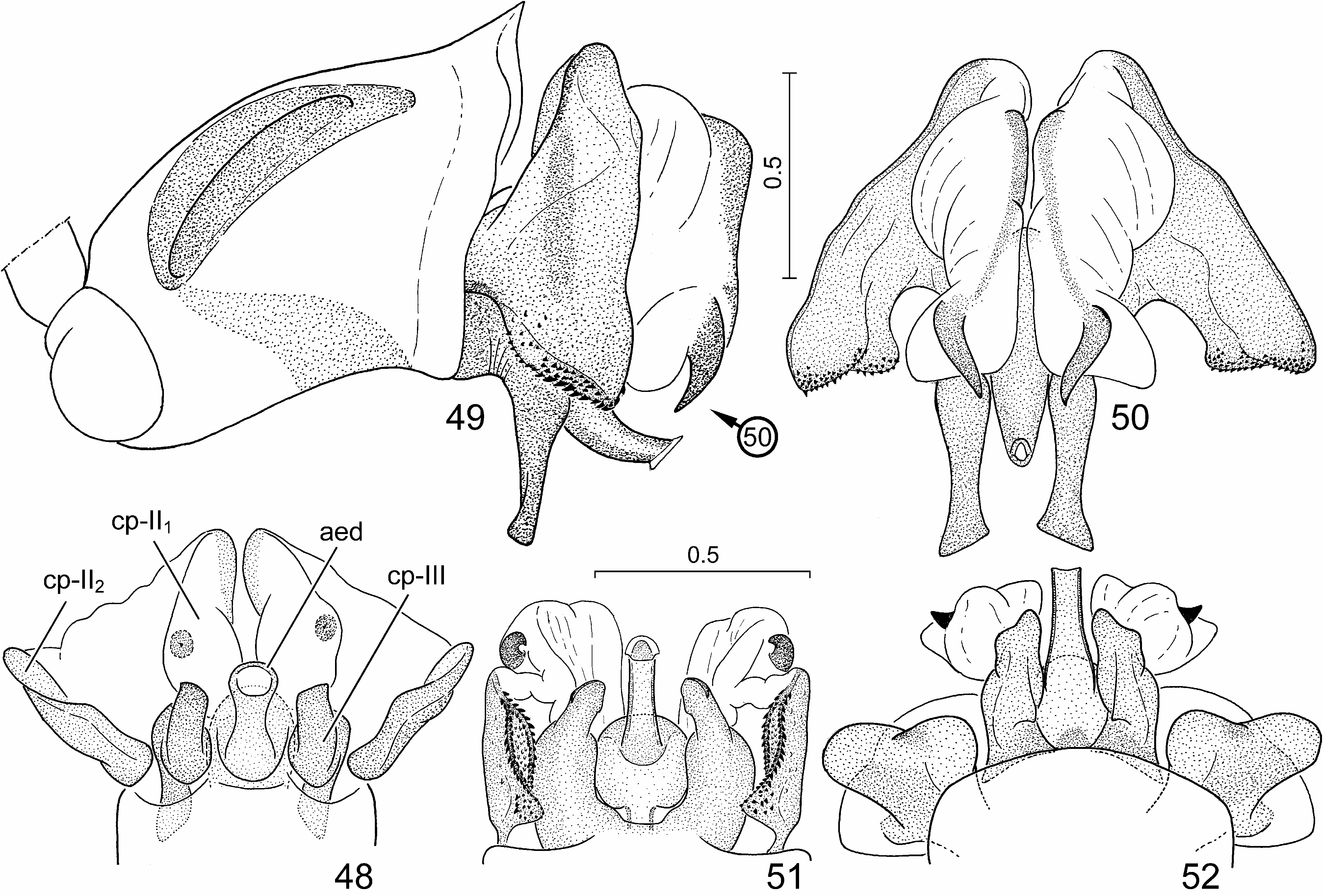
( Figs. 1–3 View FIGURES 1–10 , 11–13, 15, 16 View FIGURES 11–17 , 24, 25 View FIGURES 24–29 , 30–33 View FIGURES 30–41 , 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 , 53–56 View FIGURES 53–64 , 65, 66 View FIGURES 65–70 )
References. See under the included subspecies.
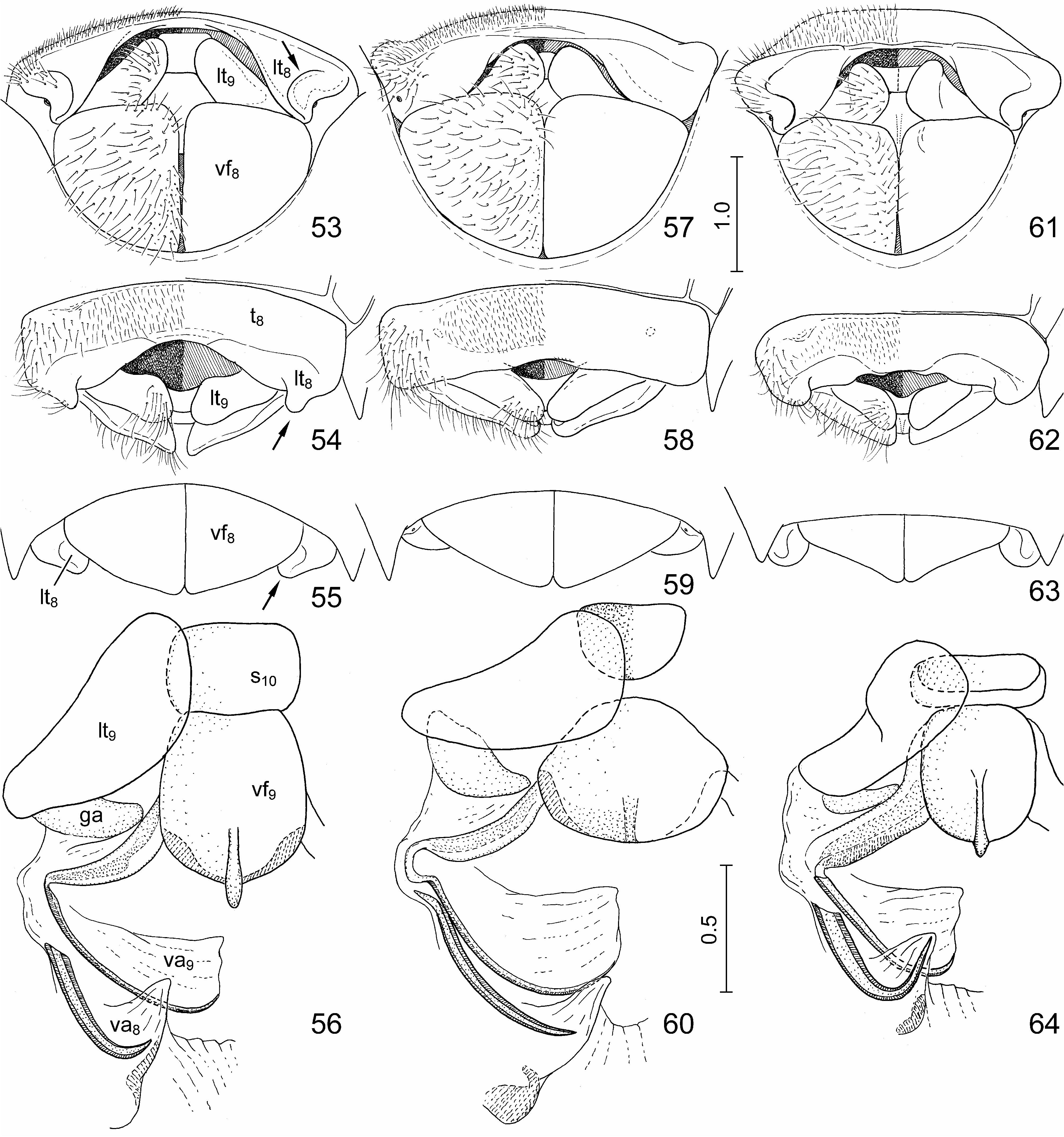
Diagnosis. Highly similar to S. perplexa , the morphology of the exoskeleton is virtually identical in the two species. In S. nepalensis the dorsal markings of the scutellum are invariably extensive, the oblique transverse fasciae always join the median vitta ( Figs. 1, 2 View FIGURES 1–10 , 11, 12, 15 View FIGURES 11–17 ); in S. perplexa the markings are highly variable ( Figs. 18, 20, 22 View FIGURES 18–23 ) but even in extreme cases the paired spots anteriad and posteriad of the middle of scutellum do not join the median vita ( Fig. 20 View FIGURES 18–23 ). The two species are most reliably separated by some characters of the terminalia of both sexes which can easily be observed even without dissection: in males of S. nepalensis the lateral margin of the genital capsule is deeply emarginate ( Figs. 24, 25 View FIGURES 24–29 , 30 View FIGURES 30–41 : arrow), but simple in S. perplexa ( Figs. 26, 27 View FIGURES 24–29 , 34 View FIGURES 30–41 ); in females of S. nepalensis the laterotergites VIII are strongly enlarged, lobe-shaped and protruding ( Figs. 53–55 View FIGURES 53–64 : arrow) whilst in S. perplexa they are much smaller, posteriorly flat ( Fig. 57–59 View FIGURES 53–64 ).
Redescription. Colour. Dorsum deep metallic blue-green ( S. nepalensis nepalensis ) ( Figs. 1, 2 View FIGURES 1–10 ) or purple ( S. nepalensis amethystina ) ( Figs. 11–13, 15, 16 View FIGURES 11–17 ), with complex and extensive black pattern; a median vitta completely occupying clypeus and extending to base of head and a pair of broad, short sublateral vittae on vertex black; mandibular plates bright orange or at least with considerable reddish or orange iridescence; antenna black, scape brownish or red at least basally; labium orange to reddish, apex of segment II darkened, segments III–IV black; pronotum broadly margined with orange or red laterally, a broad median vitta, a pair of large transverse spots on calli, a pair of large sublateral spots and a pair of small humeral spots on posterior lobe of pronotum black; scutellum with a median vitta from its base to about its middle, gradually tapering posteriad, with a pair of rounded spots at posterior part of basal tumescence, a pair of obliquely transverse fasciae anteriad to middle joining median vitta, a pair of small marginal spots at middle, a pair of broad, obliquely transverse fasciae posteriad to middle approaching or joining posterior extremity of median vitta, and a large apical spot black; exposed portion of fore wing black, its base marginally orange; venter of head and thorax metallic green usually with considerable purplish lustre, base of head posteriad to eyes, lateral margin of propleuron, anterior margin of proepisternum, greatest part of pre-, mes- and metepimeroids together with most of pro-, meso- and metathoracic supracoxal lobes orange or reddish, peritreme of metathoracic scent gland ostiole bright red; coxae, trochanters and most of femora red, an apical annulus on each femora as well as tibiae and tarsi bright metallic green; abdominal venter bright orange, ventrite II greatly black except laterally, small patches surrounding spiracles III–VII black, a pair of broad black fasciae at anterior margins of each of ventrites III–VII (medially confluent on ventrite VII) black with extensive metallic blue-green or coppery areas posteriad of them on segments III–VI; genital capsule and female terminalia greatly red, genital capsule often with a large black spot ventrally.
Body elongate, 2.3–2.4 times as long as greatest width. Body surface and vestiture as in the redescription of the genus. Pronotum relatively long, 1.6–1.7 times as broad as its median length, anterior margin broad, lateral margin nearly straight. Scutellum 1.6–1.7 times as long as broad. Membrane slightly exposed beyond apex of scutellum at rest.
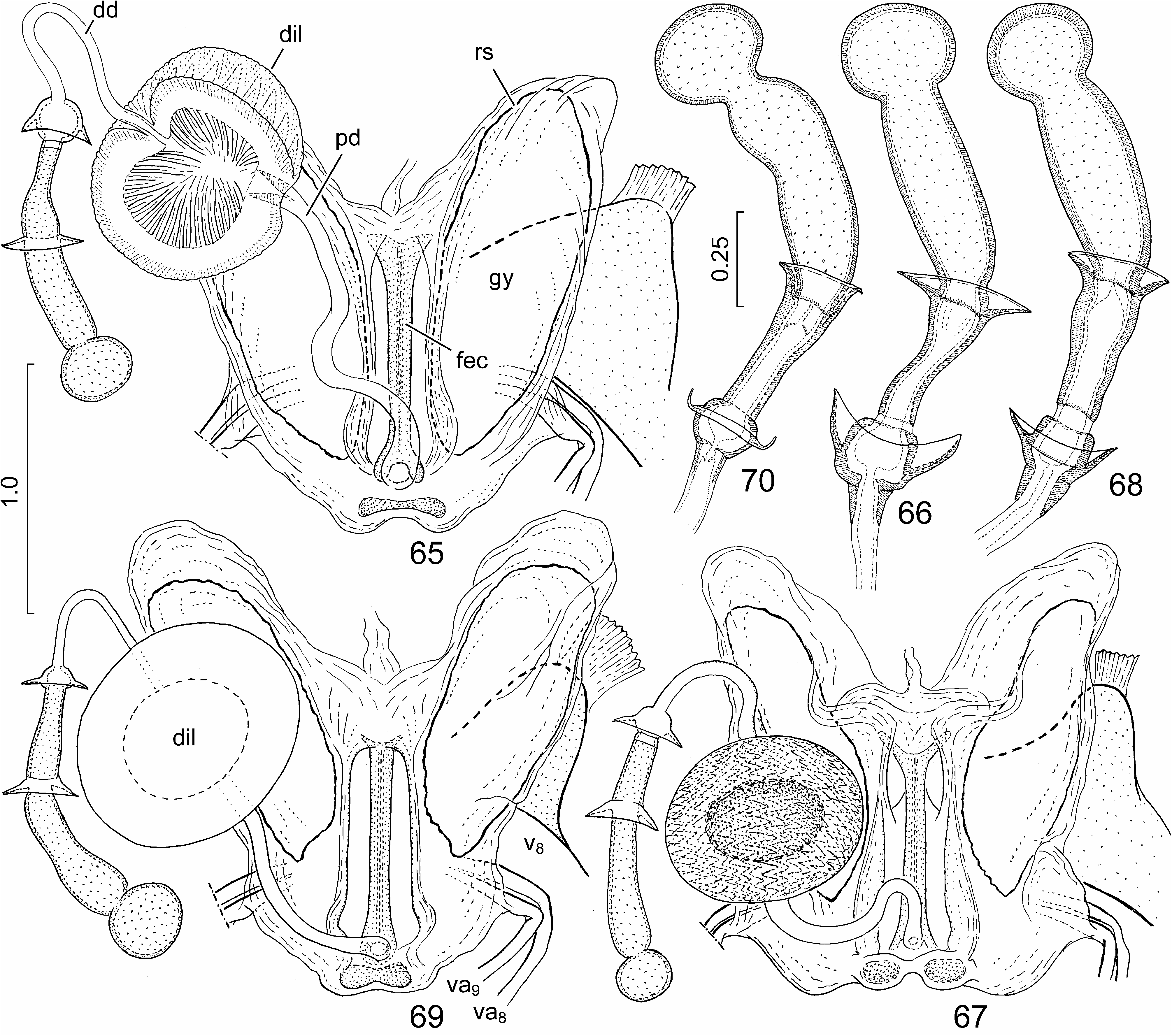
External male genitalia ( Figs. 24, 25 View FIGURES 24–29 , 30–33 View FIGURES 30–41 , 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 ) (described in detail by Tsai et al. 2011). Genital capsule ( Figs. 24, 25 View FIGURES 24–29 , 30 View FIGURES 30–41 ) subrectangular, boadly transversely truncate posteriorly in dorsal view, minute submedian denticles on posterior margin broadly separated, lateral margin (ventral rim) distinctly emarginate ( Figs. 24, 25 View FIGURES 24–29 , 30 View FIGURES 30–41 : arrow), with a pair of blunt tubercles immediately anteriad of concavity; infolding of ventral rim weakly protruding laterally, with a longitudinal ridge along meson adjacent to a pair of submedian depressions; dorsal setal patches situated along dorsal rim, ventral setal patches aside median projection of cuplike sclerite. Paramere ( Figs. 31–33 View FIGURES 30–41 ) with a relatively elongate and weakly curved crown with distal portion enclosing an acute angle with axis of stem. Phallus ( Figs. 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 ): second conjunctival processes with a small, externally partly sclerotized lateral lobe ( Figs. 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 : cp-II 1) and a large mesal lobe (cp-II 2) ( Figs. 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 : cp-II 2) terminating in a small, denticle-like sclerotized process; third conjunctival processes ( Figs. 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 : cp-III) short, irregularly rod-like, apex with a sharp longitudinal edge; distal portion of aedeagus s. str. ( Figs. 42, 43 View FIGURES 42–47 , 48 View FIGURES 48–52 : aed) and phallotreme broad.
External female genitalia ( Figs. 53–56 View FIGURES 53–64 , 65, 66 View FIGURES 65–70 ). Ovipositor. Laterotergites VIII ( Figs. 53–55 View FIGURES 53–64 : lt 8) enlarged, forming a pair of strongly protruding, rounded, lobe-like projections ( Figs. 53–55 View FIGURES 53–64 : arrow) laterad of laterotergites IX; laterotergites IX ( Figs. 53, 54 View FIGURES 53–64 : lt 9) obliquely directed, leaving sclerotized sternite X and median part of fused valvifers IX broadly exposed. Gynatrium ( Fig. 65 View FIGURES 65–70 : gy) with ring sclerites ( Fig. 65 View FIGURES 65–70 : rs) approaching apex of anterolateral pouch; small, paired sclerites posteriad of spermathecal opening fused along midline. Spermatheca: proximal duct ( Fig. 65 View FIGURES 65–70 : pd) much longer than distal duct ( Fig. 65 View FIGURES 65–70 : dd) and conspicuously longer than longitudinal diameter of dilation ( Fig. 65 View FIGURES 65–70 : dil).
Measurements (in mm). Body length to apex of scutellum 15.5–22.0; length of head 3.50–4.00, width across eyes 3.75–4.40, interocular distance 2.70–3.35; lengths of scape 0.95–1.05: basipedicellite 0.65–0.75: distipedicellite 1.45–1.90: basiflagellum 2.25–2.60: distiflagellum 2.20–2.40; median length of pronotum 4.00–5.50, humeral width 6.80–8.70; length of scutellum 10.0–13.7, greatest width 6.10–8.20.
Intraspecific variability. The ground colour of the dorsum is strongly different between the populations in Indo-China ( Fig. 1, 2 View FIGURES 1–10 ) and the Malay Archipelago ( Fig. 11, 12, 15 View FIGURES 11–17 ), but it is invariable within each population. Accordingly, two geographic subspecies, S. nepalensis nepalensis and S. nepalensis amethystina , are recognized in the present work. The variability of the black markings of the body is insignificant.
Preimaginal stages. Photos of egg batches and/or different larval instars were presented by Kanai & Sameshima (2011), Sameshima (2013) and Yiu & Yip (2012).
Habitat, bionomics, economic importance. This species was recorded from oil-seed camellia, Camellia oleifera Abel (Theaceae) ( Jiang 1985, Zhang et al. 1987) and persimmon, Diospyros kaki Thunb. (Ebenaceae) ( Xiong 1995, Lin et al. 1999) in China. In Taiwan it frequently occurs on bishop wood, Bischofia javanica Blume (Phyllanthaceae) ( Miyamoto 1965, Liu & Tseng 2005, Tsai et al. 2011); an invasive population occurring in the Ryūkyū Archipelago was observed on the same host plant ( Kanai 2010, 2013, Kanai & Sameshima 2011). Adults and larvae mainly feed on the generative parts of the host plant and frequently aggregate ( Kanai & Sameshima 2011, Sameshima 2013). Host plant records presented by Ahmad & Moizuddin (1978) and Ahmad et al. (1979) from Pakistan are based on misidentification and pertain to S. perplexa . No published data are available on its bionomics in the Malay Archipelago, but a specimen from Java examined during the present study was collected on fruits of Malay gooseberry, Phyllanthus acidus (L.) Skeels ( Phyllanthaceae ).
The life cycle of the species was described based on observations in the Ryūkyū Archipelago ( Kanai& Sameshima 2011, Sameshima 2013). Egg batches are laid on the leaves of the bishop wood, usually on the lower side, composed of 60–70 ( Kanai & Sameshima 2011, Sameshima 2013) or up to 78 (our observation) eggs arranged in two rows. Eggs hatch 12–13 days after oviposition. First instar larvae aggregate around the empty egg shells without feeding. The postembryonic development is completed in about 28 days ( Kanai & Sameshima 2011, Sameshima 2013).
Although it was recorded as a pest of persimmon in China ( Xiong 1995), it is probably of insignificant economic importance.
Remarks. This species has a long and confused nomenclatural history, summarized below.
(1) Its oldest name, Cimex amethystinus Lichtenstein, 1796 is unavailable, as it was published in a work suppressed for nomenclatural purposes (Opinion 1820) ( ICZN 1995).
(2) Cimex fasciatus Panzer, 1798 is a junior secondary homonym of Acanthia fasciata Fabricius, 1787 (junior synonym of Cimex (now Anthocoris ) nemorum Linnaeus, 1761, Anthocoridae ) subsequently combined with the generic name Cimex Linnaeus, 1758 by Villers (1789: 398) and Gmelin (1790: 2125) (cf. Dolling et al. 1999: 33). In spite of this homonymy this scutellerid species has commonly been referred to in the literature in the combination Scutellera fasciata . The relevant taxa have not been considered congeneric since about 1814. Kirkaldy (1909) explicitly treated C. fasciatus Panzer, 1798 as preoccupied and treated Scutellera amethystina ( Germar, 1839) as the valid name of this species. This act is an explicit replacement ( ICZN 1999, Art. 60) of the junior secondary homonym with an available and potentially valid synonym, therefore Cimex fasciatus is permanently invalid ( ICZN 1999, Art. 59.3).
(3) Tectocoris nepalensis Westwood, 1837 was synonymized with S. fasciata by Dallas (1851) and subsequently it has never been cited as valid except of Dolling et al. (1999: 7).
(4) Calliphara amethystina Germar, 1839 is a junior secondary homonym and subjective junior synonym of Cimex amethystinus Lichtenstein, 1796 , but as the latter is unavailable, it does not take precedence over the junior name. The name was used by Kirkaldy (1909) and several subsequent authors as the valid name of the biological species in concern, but this practice is erroneous, as this name is pre-dated by Tectocoris nepalensis Westwood, 1837 .
Dallas (1851) recognized all the above species as conspecific; all subsequent authors followed this interpretation. Based on the re-examination of the available types and dissection of several specimens from southeastern Asia ( China, Taiwan, and Indonesia: Java) we concur with Dallas (1851) in recognizing them as conspecific. However, as the population in continental Asia and in the Malay Archipelago sharply and constantly differ in colour, and no transitional specimens were seen, we recognize them as two geographic subspecies. The lectotype of Tectocoris nepalensis pertains to the former one, that of Calliphara amethystina to the latter. As T. nepalensis is an available name, following Dolling et al. (1999) we recognize it as the valid name of the species, and downgrade C. amethystina to subspecies rank.
Most of the previous authors used Scutellera amethystina as the valid name of this species, without differentiating subspecies. Previous literature records of S. amethystina therefore might pertain either to S. nepalensis nepalensis or S. nepalensis amethystina , or frequently to both of them. Such references are listed under the nominotypical subspecies in the literature reviews below.
Tectocoris nepalensis was described based on an unspecified number of specimens (syntypes) (Westwood 1837). The single syntype deposited in OXUM ( Figs. 1–4 View FIGURES 1–10 ) was erroneously listed as the holotype of the unrelated species Poecilocoris nepalensis (Herrich-Schäffer, 1837) by Ahmad & Kamaluddin (1982), thus effectively designating it as the lectotype of the latter ( ICZN 1999, Art. 74.6), however, invalidly, because the specimen in concern certainly cannot be considered as a syntype of that species ( ICZN 1999, Art. 74.2). The same specimen is hereby designated as the lectotype of Tectocoris nepalensis Westwood, 1837 . Göllner-Scheiding (2006) claimed that the syntype (s) of Calliphara amethystina were lost; however, four specimens deposited in the ZMHB were examined during the present study which likely represent syntypes, and one of them is designated as lectotype.
The specimen figured by Lin et al. (1999: 47, fig. 15-85) identified as S. fasciata is apparently S. perplexa . The specimen in the photograph of Ho (2003: 195) identified as S. amethystina and the painting of Cai & Li (2015: 157) purportedly showing S. fasciata both represent Brachyaulax cyaneovitta ( Walker, 1867) . The specimen photographed by Parveen & Gaur (2015: 181) as S. fasciata represents Tetrarthria variegata Dallas, 1851 .
A partial 16S rRNA gene sequence for S. nepalensis nepalensis (as S. amethystina ) was provided by Hsieh et al. (2019) and it is available from GenBank (accession no. HG810206.1).
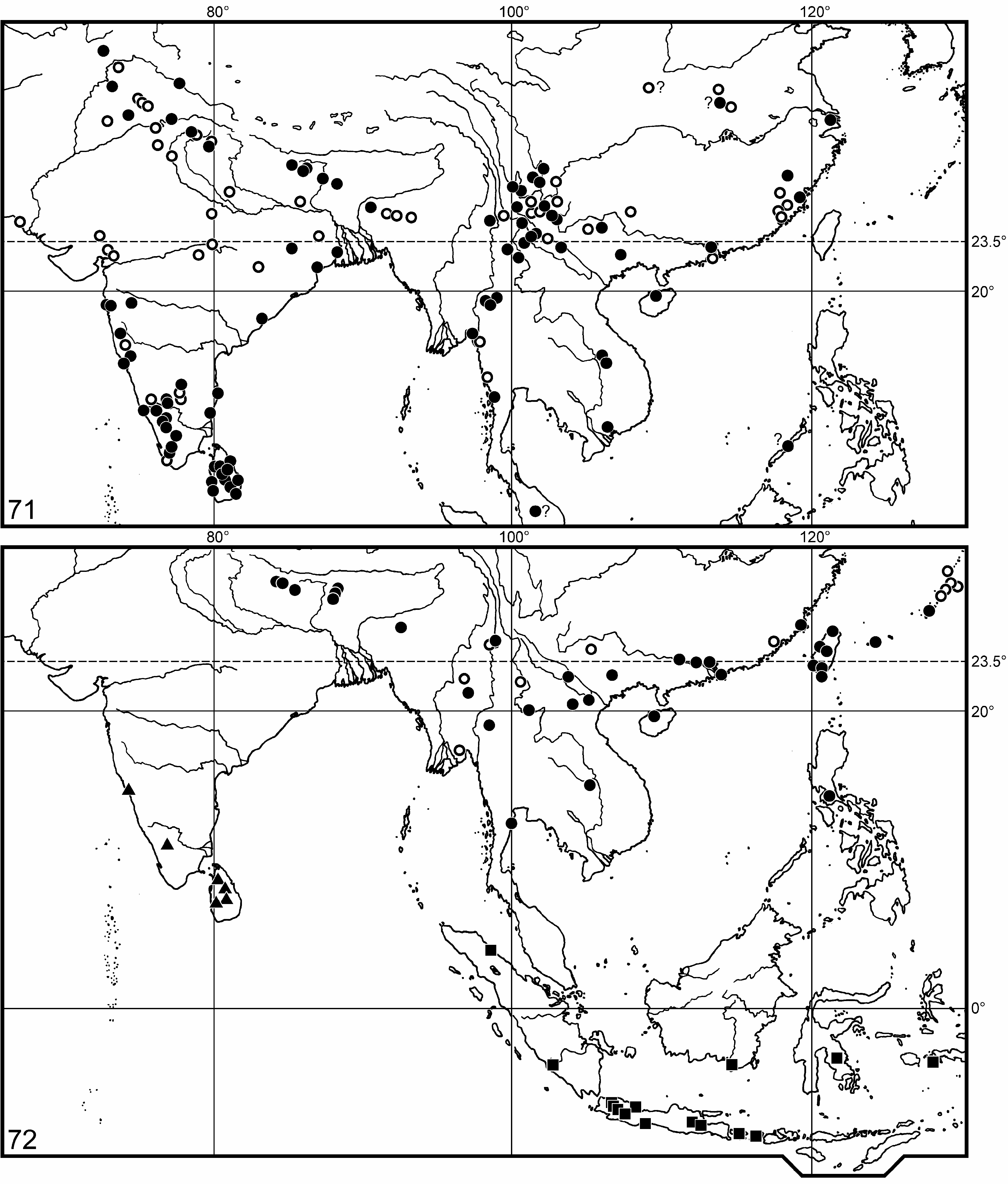
Distribution. Scutellera nepalensis is widely distributed in the Sub-Himalayan belt, South China, Indo-China, Taiwan, the Ryūkyū Archipelago, and the Malay Archipelago ( Fig. 72 View FIGURES 71–72 ); see also under its two subspecies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.