Nymphargus lasgralarias Hutter & Guayasamin
|
publication ID |
https://doi.org/ 10.5281/zenodo.280678 |
|
DOI |
https://doi.org/10.5281/zenodo.5628874 |
|
persistent identifier |
https://treatment.plazi.org/id/03EFEB76-FFCD-FFB2-0481-FD20FAA0FD03 |
|
treatment provided by |
Plazi |
|
scientific name |
Nymphargus lasgralarias Hutter & Guayasamin |
| status |
sp. nov. |
Nymphargus lasgralarias Hutter & Guayasamin View in CoL , new species
Figures 4 View FIGURE 4 A–4C; 5B; 6A–6D; 7A–7D; 13D
Holotype. MZUTI 0 96, adult male collected by Carl R. Hutter on 0 5 April 2011 from “Five Frog Creek” (0º01.870’ S, 78º42.358’ W; 2150 m) at Reserva Las Gralarias, Pichincha province, Ecuador. Figure 5 View FIGURE 5 B.
Paratypes. MZUTI 091–095, and 0 97, adult males obtained from Reserva Las Gralarias by Carl R. Hutter. MZUTI 093–095 were collected on 0 5 April 2011; MZUTI 0 92 on 17 April 2011; and MZUTI 0 91 on 18 April 2011 from “Kathy’s Creek” (0º01.398’ S, 78º43.772’ W; 2000 m). MZUTI 0 97 was collected on 0 1 July 2011 from “Hercules Giant Tree Frog Creek” (0º01.529’ S, 78º42.243’ W; 2175 m).
Generic placement. All species in Nymphargus share an absence of webbing among Fingers I–III and absence or reduced webbing between Fingers III and IV. Additionally, males lack humeral spines (except N. grandisonae ). The new species presents the aforementioned traits and, therefore, is placed in Nymphargus (sensu Guayasamin et al. 2009).
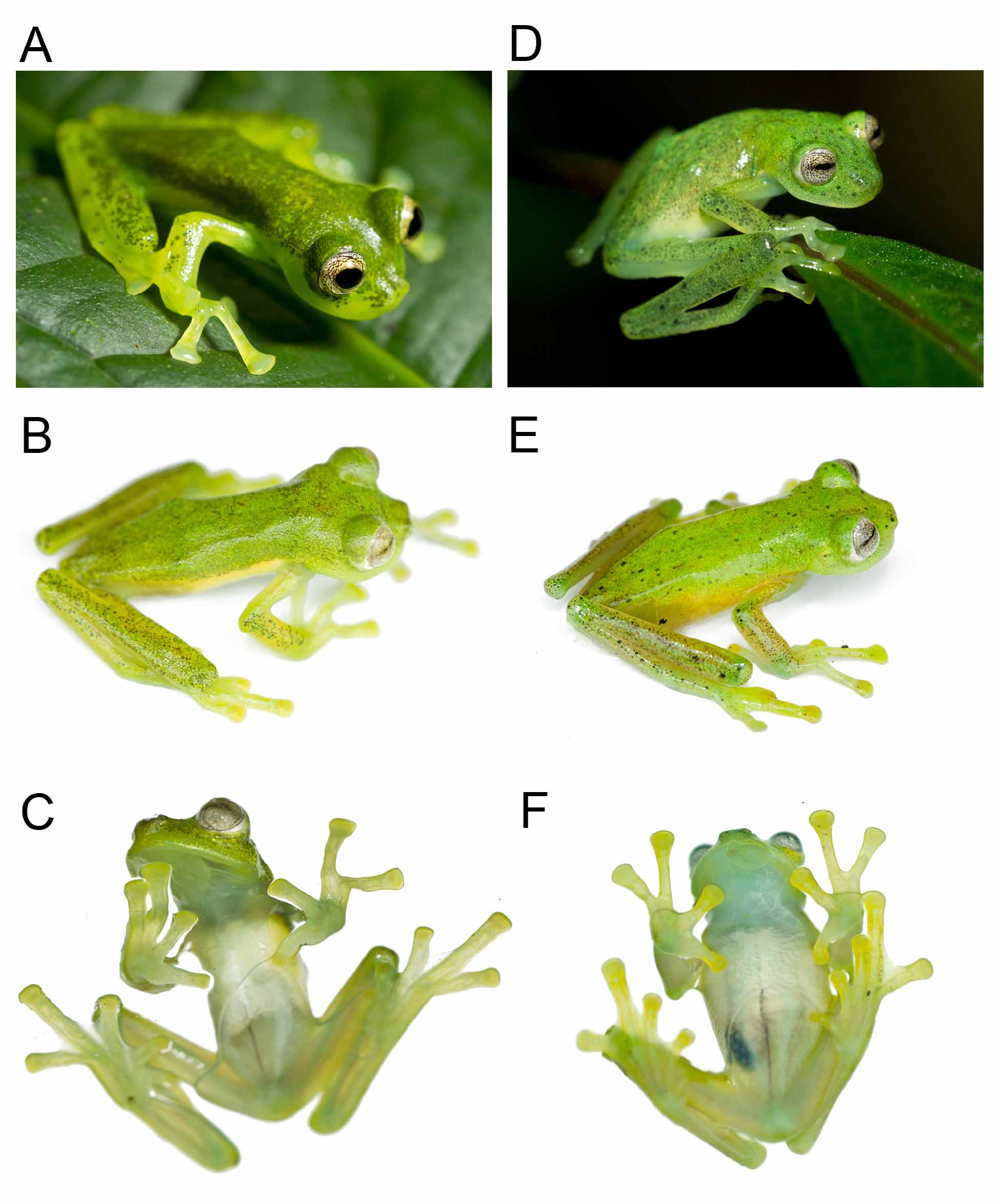
Diagnosis. The new species can be distinguished from most species of Nymphargus by having a uniformly green dorsum (see Guayasamin et al. 2009). Within Nymphargus , the only species with a green dorsum that lacks spots are: N. cristinae ( Ruiz-Carranza & Lynch 1995) , N. prasinus ( Duellman 1981) , and N. wileyi ( Guayasamin et al. 2006) . Nymphargus lasgralarias sp. nov. is distinguished from N. cristinae by being smaller (male SVL in N. lasgralarias = 24.6–26.5 mm [mean = 25.3 mm; n = 7]; male SVL in N. cristinae = 26.0– 31.1 mm [mean = 28.0 mm; n = 12]), having a snout that is truncate in dorsal view and protruding in lateral view (subacuminate in dorsal view, truncate in lateral view in N. cristinae ; see Ruiz-Carranza & Lynch 1995: Fig. 4 View FIGURE 4 ), lacking vomerine teeth (present or absent in N. cristinae ), and lacking palmar supernumerary tubercles (supernumerary small, abundant in N. cristinae ). Nymphargus prasinus differs from N. lasgralarias sp. nov. by having a round snout in dorsal view (truncate N. lasgralarias sp. nov.), 5–7 teeth on each process of the vomer (vomerine teeth absent in N. lasgralarias sp. nov.), and being considerably larger (male SVL 33.0– 34.5 mm; n = 3; see Duellman 1981). Nymphargus wileyi (an endemic of the Amazonian slopes of the Ecuadorian Andes) is distinguished from N. lasgralarias sp. nov. by having its kidneys covered by a white peritoneum with small, unpigmented spots (see Guayasamin et al. 2006: Fig. 12 View FIGURE 12 ), whereas in the new species, the kidneys are covered by a homogenously white layer. Additionally, among Nymphargus species found on the Pacific versant of the Andes of Ecuador, Nymphargus lasgralarias sp. nov. could only be confused with N. buenaventura ( Cisneros-Heredia & Yánez-Muñoz 2007) and N. griffithsi ( Goin 1961) . Dorsal texture and color pattern readily separates N. buenaventura , which, in life, has a light green dorsum with warts corresponding to pale yellow spots — whereas the dorsum of N. lasgralarias sp. nov. is shagreen (lacking warts) and homogenously green (lacking yellow spots). Additionally, N. buenaventura is smaller, although sample size is low (male SVL in N. lasgralarias sp. nov. = 24.6–26.5 mm [mean = 25.3 mm; n = 7]; male SVL in N. buenaventura = 20.9–22.4 mm [mean = 21.8; n = 4]). Nymphargus lasgralarias sp. nov. and N. buenaventura are not known to occur sympatrically.
Nymphargus lasgralarias sp. nov. is most similar to N. griffithsi . However, the two species have differences in terms of dorsal color pattern (homogenously green with minute dark melanophores in N. lasgralarias sp. nov.
[ Figs. 4 View FIGURE 4 B, 6A–6B]); green with small black spots and/or both minute and small dark melanophores in N. griffithsi [ Figs. 4 View FIGURE 4 E, 6E–6F]), body size (male SVL in N. lasgralarias sp. nov. = 24.6–26.5 mm [mean = 25.3 mm; SD = 0.73 mm; n = 7]; male SVL in N. griffithsi = 22.5–24.2 mm [mean = 23.0 mm; SD = 0.70 mm; n = 5]; T-test: p <0.001), and call (see Advertisement call section). Additionally, in life, N. griffithsi has an iris background coloration of white-silver with larger and less abundant spotting with some medium-dark reticulation ( Fig. 7 View FIGURE 7 E–7H), whereas N. lasgralarias sp. nov. has a yellow-golden iris background color with lighter reticulation and more numerous, smaller spots ( Fig. 7 View FIGURE 7 A–7D).
Characterization. (1) Vomerine teeth absent; (2) snout truncate in dorsal profile, protruding in lateral profile; (3) tympanum small; supratympanic fold present; tympanic membrane translucent, pigmented only on its upper half; (4) skin texture finely shagreen, with microspiculations; (5) ventral skin areolate, with pair of large, round warts on ventral surfaces of thighs below vent; cloaca surrounded by low warts, non-enameled; (6) upper half of ventral parietal peritoneum covered by iridophores (= white), all other peritonea translucent, except for thin layer of iridophores covering heart and renal capsules; (7) liver tetralobed; (8) humeral spines absent; (9) webbing absent between fingers; (10) foot about half webbed; webbing formula: I (2–2–) — (2+–2 1/2) II (2–2–) — (3– –3) III (2– –2) — (3– –3) IV (3–3+) — 2 V; (11) ulnar and tarsal folds low, barely evident, non-enameled; (12) nuptial pad Type I; prepollex not separated from Finger I; (13) first finger slightly shorter than second; (14) eye diameter larger that width of disc on Finger III; (15) in life, green dorsum, with minute dark melanophores; (16) in preservative, dorsum pale lavender; (17) iris golden-yellow, with numerous small black spots; weakly reticulated; (18) hands and feet yellowish green; melanophores absent from fingers and toes or, when present, restricted to dorsal surfaces of Finger IV and Toes IV and V; (19) males call from the upper side of leaves along streams; (20) calls emitted in series of 1–4 calls; each call sounding like a “tick” or “click”; pulsed; duration of 0.0160– 0.0440 s (mean = 0.0257 ± 0.0058; n = 119); call non-modulated to weakly modulated; dominant frequency at 3445.3–3962.2 Hz (mean = 3691.4 ± 131.9 Hz); (21) fighting behavior unknown; (22) egg clutches deposited on upper surface of leaves at terminal margin, transitioning to hanging as eggs develop; (23) tadpoles unknown; (24) SVL in adult males 24.6–26.5 mm (mean = 25.3 ± 0.737; n = 7); females unknown.
Description of holotype. MZUTI 0 96, adult male, SVL 25.5 mm. Head wider than long; head length 32% SVL; snout truncate in dorsal profile, protruding in lateral view; canthus rostralis indistinct, straight; loreal region slightly concave; lips slightly flared; nostrils protuberant, closer to tip of snout than to eye, directed dorsolaterally; internarial area barely depressed. Eye large, directed anterolaterally at an angle of 45°; transverse diameter of disc of Finger III 57.6% eye diameter. Supratympanic fold conspicuous, obscuring dorsal portion of tympanic annulus; tympanum small (3% of SVL), oriented mostly vertically, but with slight posterolateral inclination; tympanic membrane transparent, partially pigmented and differentiated from surrounding skin. Dentigerous processes of vomer low, situated transversely between choanae, lacking teeth; choanae large, longitudinally rectangular; tongue ovoid, with ventral posterior third not attached to mouth floor and posterior margin notched; vocal slits extending posterolaterally from the lateral edge of tongue to angle of jaws. Humeral spine absent; ulnar fold low, barely evident, nonenameled; relative lengths of fingers: III> IV> II> I; webbing between fingers absent; discs expanded, nearly round; disc pads triangular; subarticular tubercles small, round, simple; few palmar supernumerary tubercles evident, low; palmar tubercle elliptical, simple; nuptial pad Type I (sensu Flores 1985), ovoid, granular, extending from ventrolateral base to dorsal surface of Finger I, covering the proximal half of Finger I. Length of tibia 56% SVL; low inner tarsal fold barely evident; outer tarsal fold absent; feet about half webbed; webbing formula of foot: I 2 – — 2 1/ 2 II 2– — 3 III 2– –– 3– IV 3— 2 V; discs on toes round; disc on Toe IV narrower that disc on Finger III; disc pads triangular; inner metatarsal tubercle large, ovoid; outer metatarsal tubercle round, barely evident; subarticular tubercles small, round; supernumerary tubercles absent.
Skin on dorsal surfaces of head, body, and lateral surface of head and flanks shagreen with numerous minute spinules; throat smooth; belly and lower flanks areolate; cloacal opening directed posteriorly at upper level of thighs; cloacal warts small, fleshy, located immediately posterior to cloacal slit, non-enameled. Pair of large subcloacal tubercles evident in ventral aspect.
Measurements of holotype. Morphometrics of the holotype and paratypes are summarized in Table 1 View TABLE 1 .
Nymphargus lasgralarias sp. nov.
Color in life. Dorsum light green, with minute melanophores; flanks yellowish white; bones green; fingers and toes yellow with a faint green tint. Venter white anteriorly and translucent posteriorly. Iris background golden with numerous dark spots and very light reticulation.
Color in preservative. Dorsal surfaces of head and body are cream; fingers and toes cream. Upper half of ventral parietal peritoneum covered by iridophores (= white), all other peritonea translucent, except for thin layer of iridophores covering heart and renal capsules.
Variation. In life, dorsal coloration varies from very light green to light green. Coloration of dorsum in preservation varies from cream to medium-dark lavender. Females are unknown.
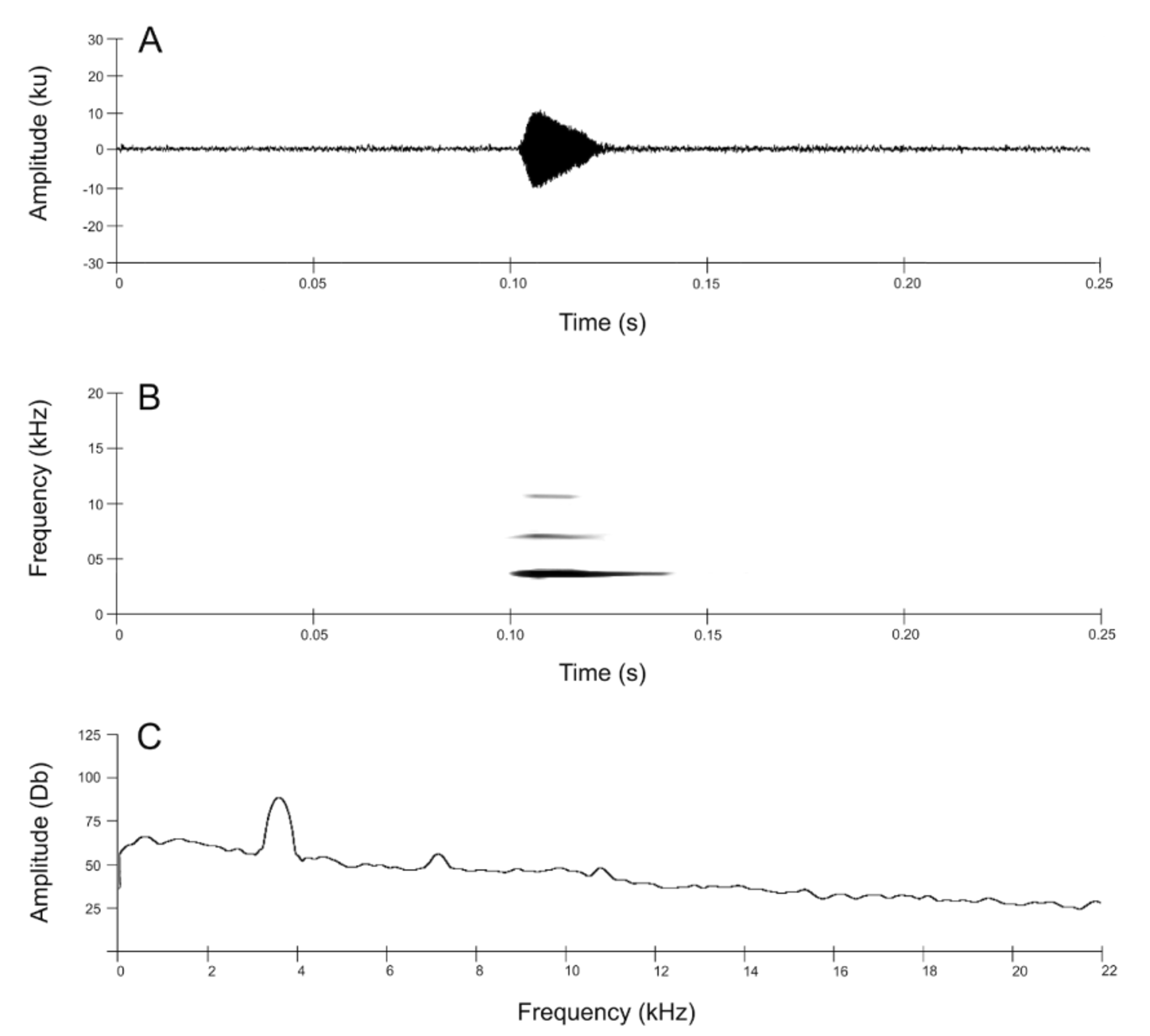
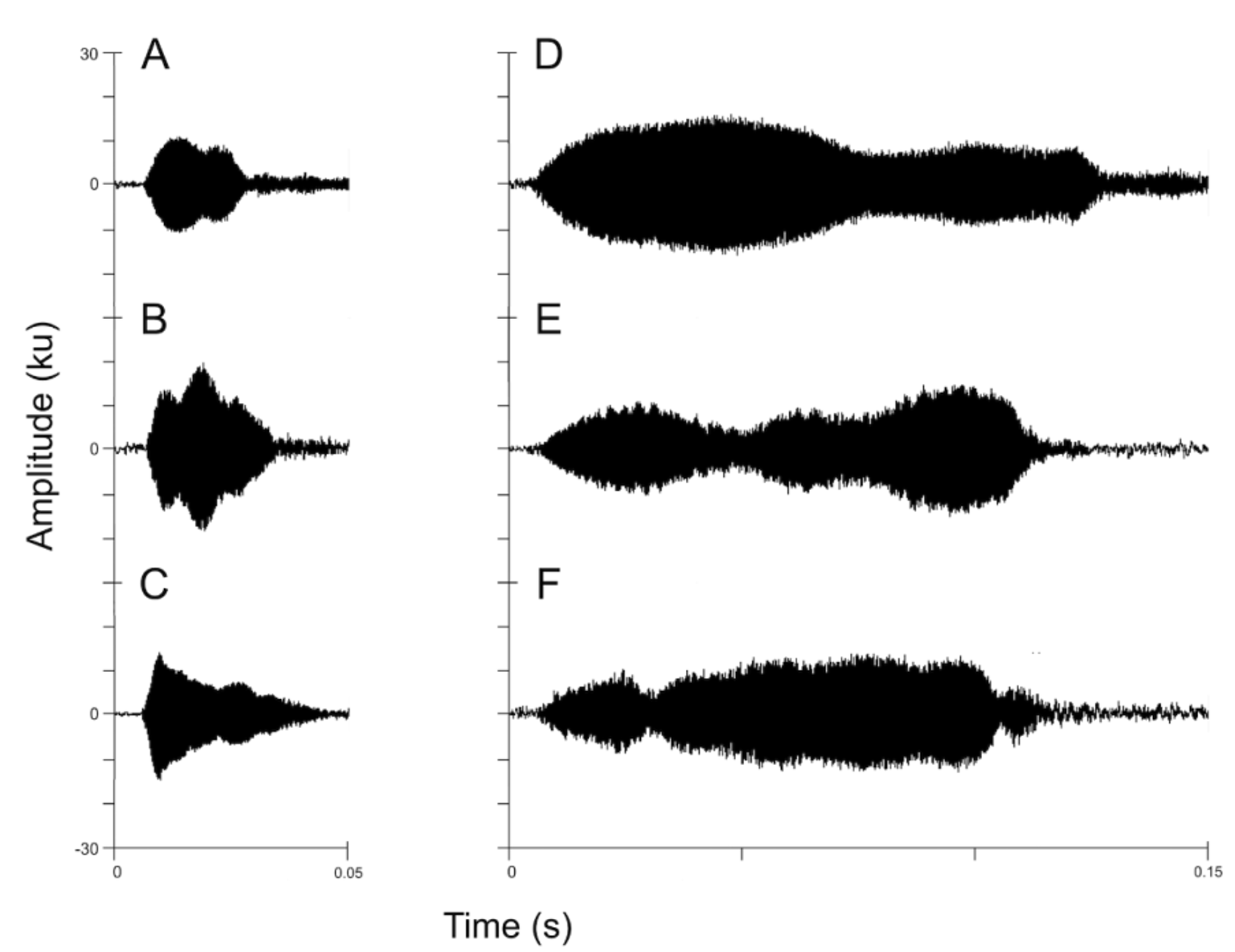
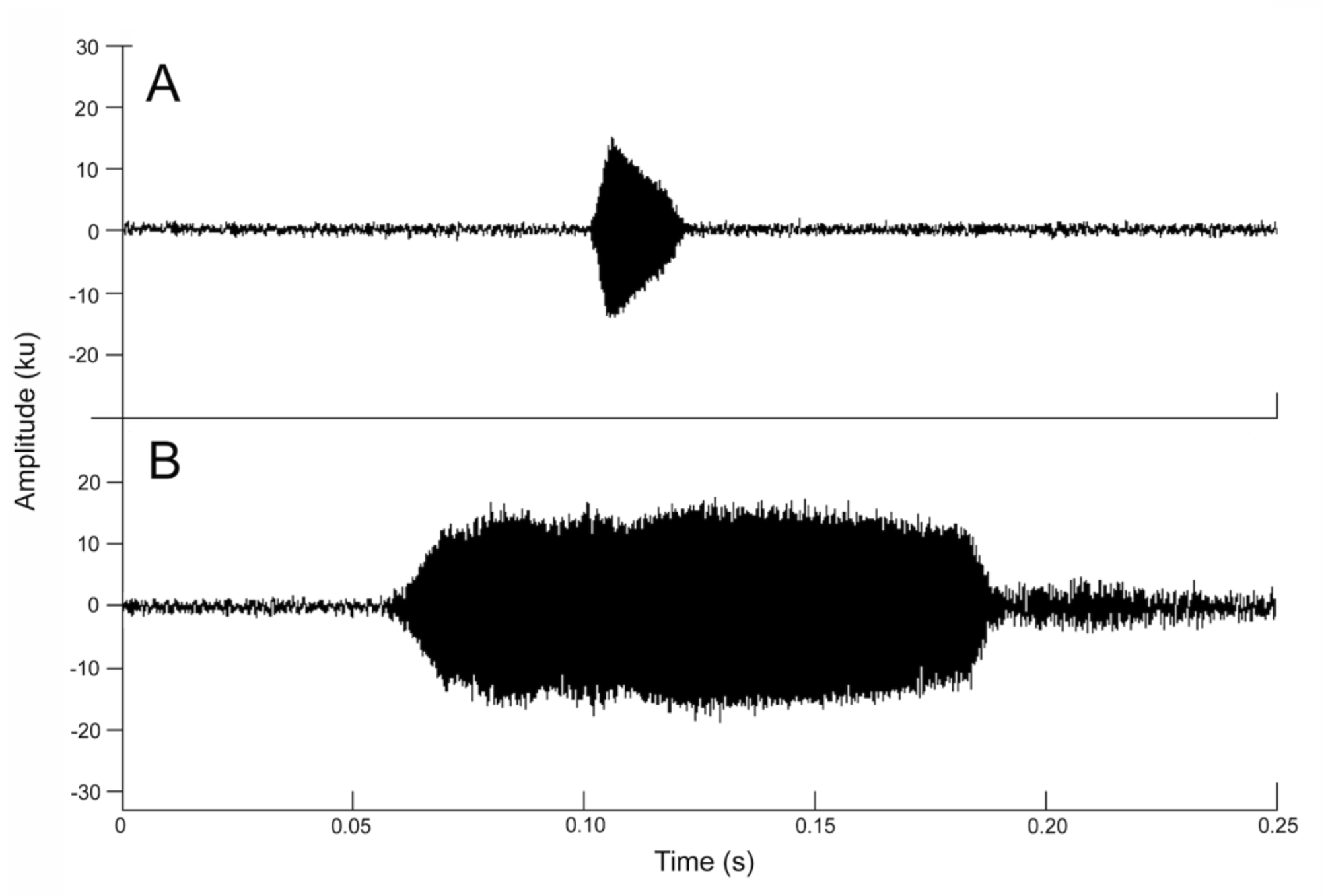
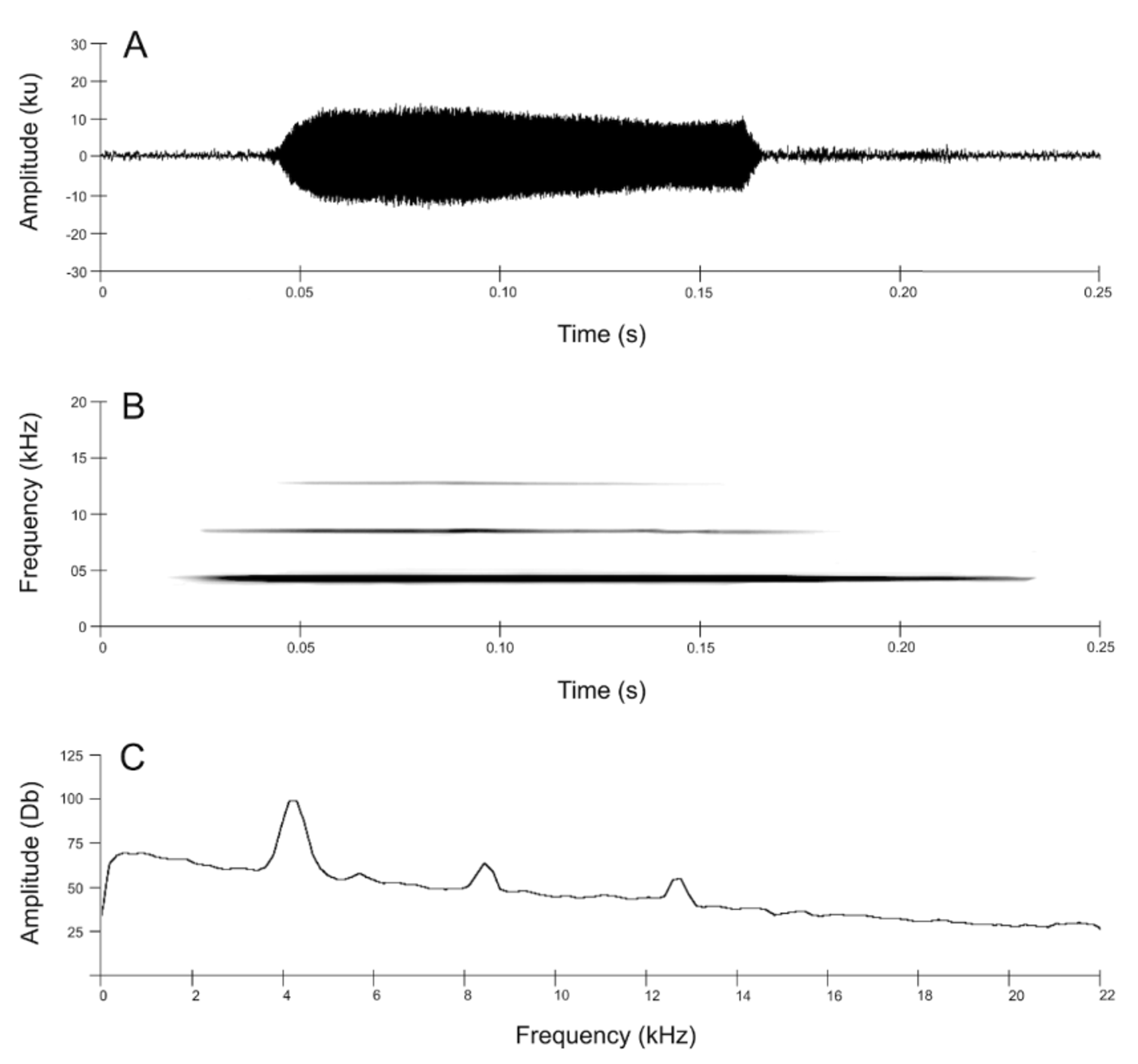
Advertisement Call. The call of Nymphargus lasgralarias sp. nov. is reminiscent of a short “ticking” or “clicking” noise and is easily distinguishable from the significantly longer “whistle” produced by Nymphargus griffithsi ( Figs. 8–10 View FIGURE 8. A View FIGURE 9 View FIGURE 10 ). The call consists of a short, pulsed note lasting 0.016– 0.044 s (mean = 0.026 ± 0.006 s) with 1–3 pulses (mean = 1.5 ± 0.6 pulses) ( Figs. 9 View FIGURE 9 , 11 View FIGURE 11 A–11C). Calls emitted in a series, which typically includes 1–4 calls (mean = 2.7 ± 0.7 calls) ( Fig. 12 View FIGURE 12 A–12D). Five-call series had been observed, but were not recorded. Each series has duration of 0.033– 2.541 s (mean = 1.529 ± 0.597 s) and an interval of 8.6– 78.6 s (mean 33.8 ± 18.4 s) between series with an interval of 0.088– 1.513 s (mean = 0.873 ± 0.205 s) between calls within a series. The call repetition rate is 2.0–9.9 (5.5 ± 2.7) calls per minute (n = 6 individuals). The dominant frequency is measured at 3445.3–3962.2 Hz (mean = 3691.4 ± 131.9 Hz); contained within the fundamental frequency. The individual call begins at an initial fundamental frequency of 2561.0–3441.0 Hz (mean 3063.6 ± 162.5 Hz). The fundamental frequency is bound between the lower frequency of 2939.4–4145.2 Hz (mean = 3236.3 ± 168.7) and the upper frequency of 3887.7–4473.4 Hz (mean = 4139.8 ± 139.7 Hz). The call has three harmonic frequencies at 6546.1– 8096.5 Hz (mean = 7298.9 ± 305.8 Hz), 9991.4–12058.6 Hz (mean = 11034.2 ± 478.3 Hz), and 13781.2–14928.0 Hz (mean 14317.1 ± 245.5 Hz).
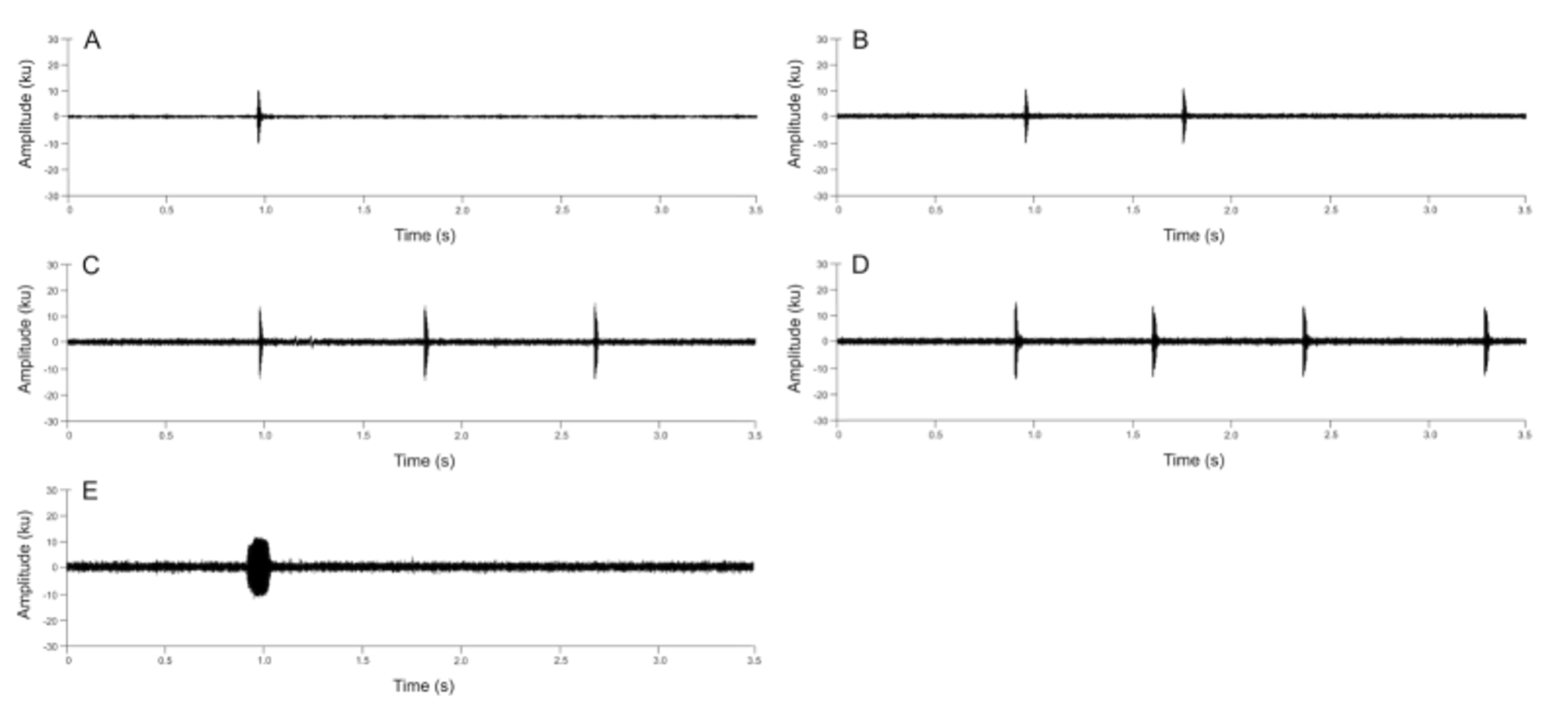
A quantitative comparison between the calls of Nymphargus lasgralarias sp. nov. and Nymphargus griffithsi is shown in Table 2 View TABLE 2. A . Structurally, the calls of the two species are quite different ( Fig. 8 View FIGURE 8. A ). The call of N. griffithsi is a single tonal or multi-pulsed (i.e., 2 or more pulses) call ( Figs. 8 View FIGURE 8. A B, 10, 11D–11F), whereas the calls of N. lasgralarias sp. nov. are always pulsed ( Figs. 8A View FIGURE 8. A , 9 View FIGURE 9 , 11 View FIGURE 11 A–11C). Nymphargus griffithsi emits its advertisement call as a single call (absent from a series) while N. lasgralarias sp. nov. emits its calls as a single call or in a series, demonstrating a highly variable calling pattern in contrast to N. griffithsi ( Fig. 12 View FIGURE 12 A–12D). In addition, N. lasgralarias sp. nov. has a significantly shorter call duration than N. griffithsi (call duration in N. lasgralarias sp. nov. = 0.016– 0.044 s [mean = 0.026 s; SD = 0.006 s; n = 119]; call duration in N. griffithsi = 0.103– 0.148 s [mean = 0.122 s; SD = 0.009 s; n = 48]; T-test: p <0.001).
The dominant frequency is significantly lower in N. lasgralarias sp. nov. than N. griffithsi (dominant frequency in N. lasgralarias sp. nov. = 3445.3–3962.2 Hz [mean = 3691.4 Hz; SD = 131.9 Hz; n = 119]; dominant frequency in N. griffithsi = 3789.8–4306.6 Hz [mean = 4107.4 Hz; SD = 105.5 Hz; n = 48]; T-test: p <0.001), although there is partial overlap. Although the calls of N. lasgralarias sp. nov. and N. griffithsi do not show a conspicuous change in dominant frequency, the two species show a slight increase in the dominant frequency, an increase that is more pronounced it N. griffithsi . Furthermore, N. lasgralarias sp. nov. has a significantly lower initialization frequency (Hz) than N. griffithsi (initial frequency in N. lasgralarias sp. nov. = 2561.0–3441.0 Hz [mean = 3063.6 Hz; SD = 162.5 Hz; n = 119]; initial frequency in N. griffithsi = 2821.0–3776.0 Hz [mean = 3328.6 Hz; SD = 300.9 Hz; n = 48]; T-test: p <0.001). A quantitative comparison between the calls of N. lasgralarias sp. nov. and N. griffithsi is shown in Table 2 View TABLE 2. A . Additional detailed acoustic measurements can be found in APPENDIX II and APPENDIX III.
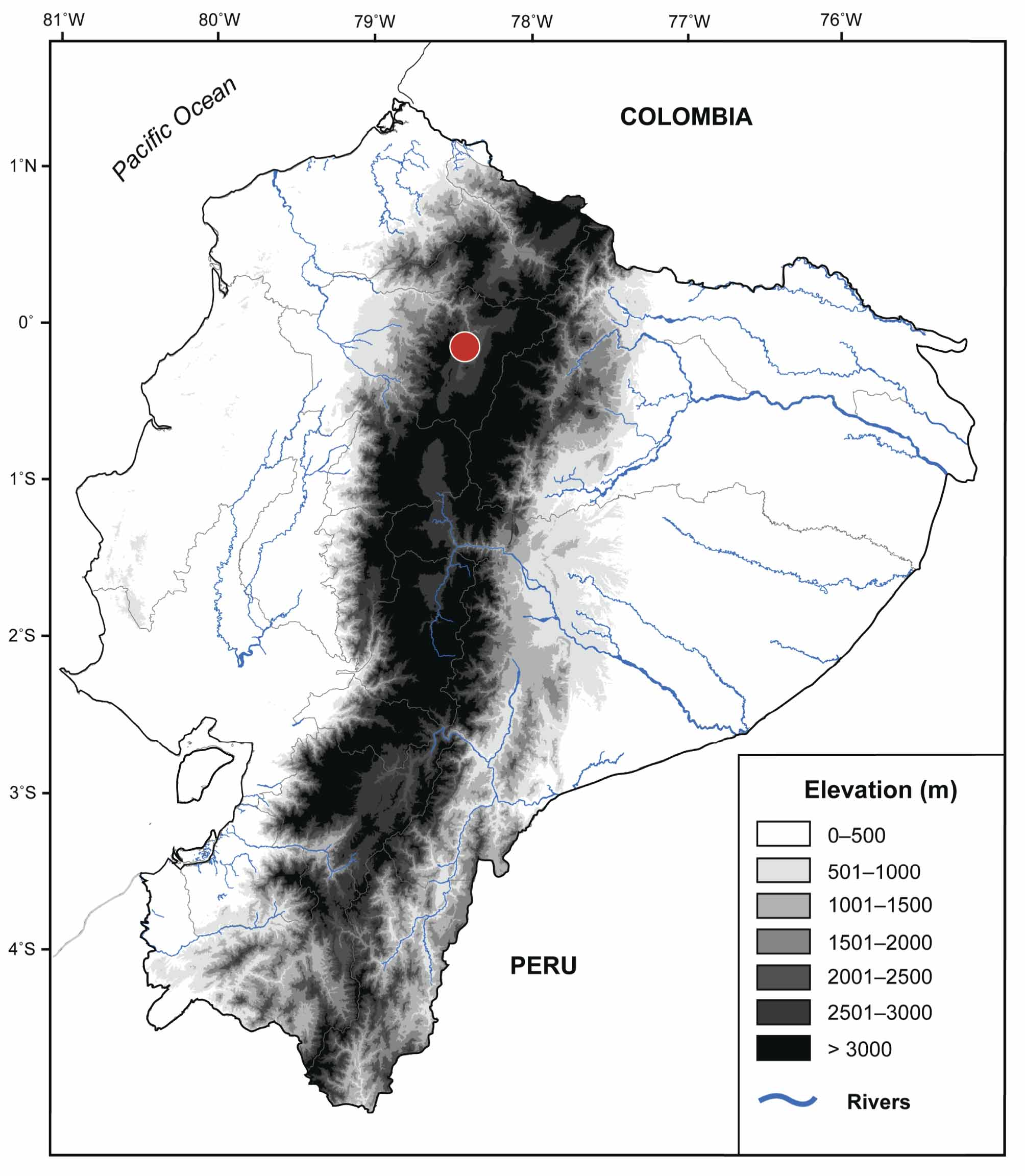
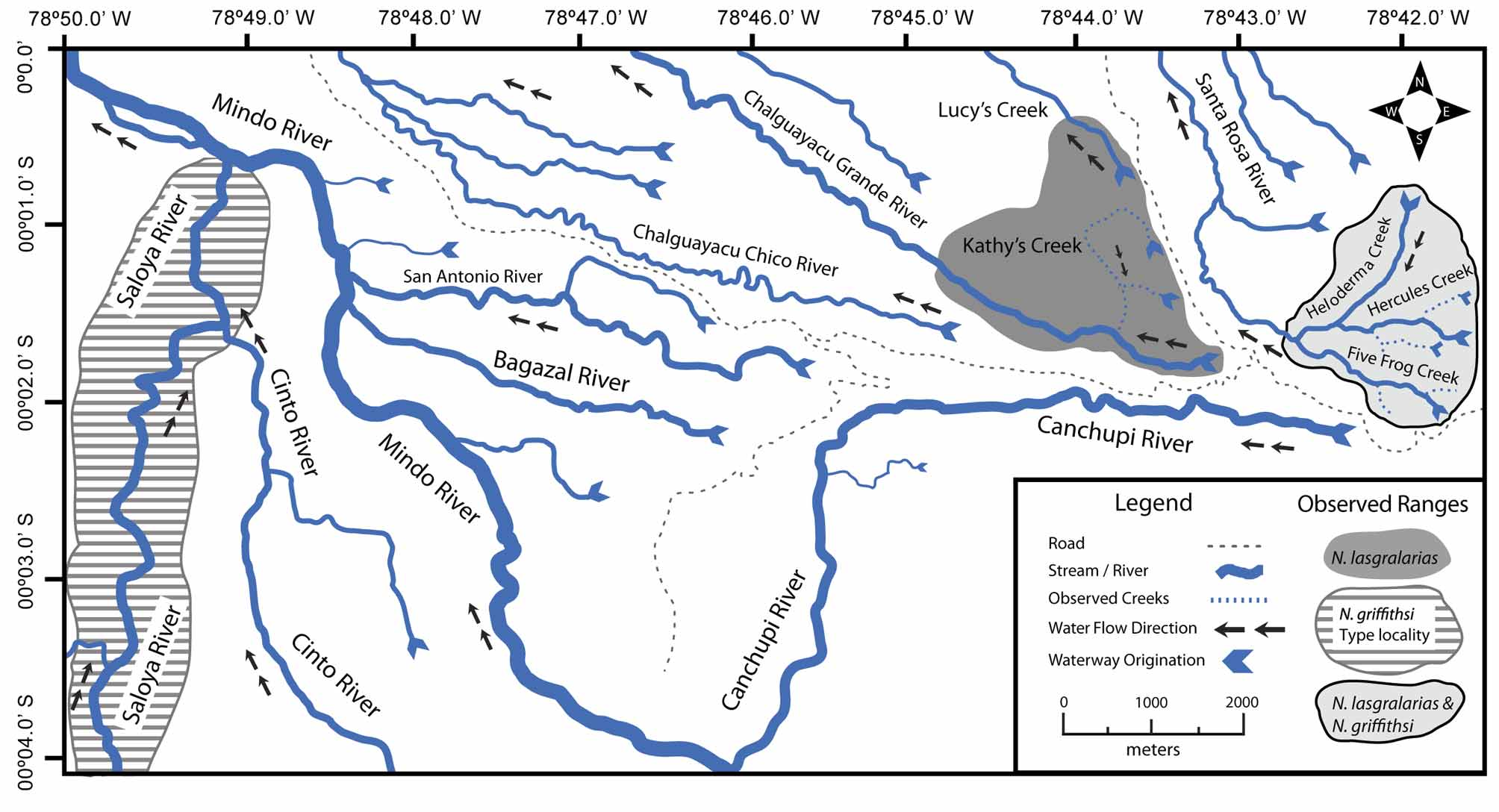
Distribution. Nymphargus lasgralarias sp. nov. is known only from its type locality at Reserva Las Gralarias ( Fig. 1 View FIGURE 1 ) in Pichincha province, Ecuador, between an elevation of 1850–2200 m. Within the reserve, N. lasgralarias sp. nov. is known from the Chalguayacu Grande River (0º01.868’ S, 78º44.057’ W; 1925–2000 m), “Five Frog Creek”, “ Heloderma Creek” (0º01.245’ S, 78º42.370’ W; 2175–2225 m), “Hercules Giant Tree Frog Creek”, “Kathy’s Creek”, and “Lucy’s Creek” (0º00.585’ S, 78º43.901’ W; 1850–1875 m). Nymphargus lasgralarias sp. nov. is quite ubiquitous throughout Reserva Las Gralarias, with observations only absent from the Santa Rosa River (0º01.192’ S, 78º43.212’ W; 1825–1850 m) ( Table 3; Fig. 2 View FIGURE 2 ).
Species
N. lasgralarias sp. nov. N. griffithsi
Parameter Range Mean ± SD Range Mean ± SD Species
Centrolene ballux Centrolene lynchi Centrolene peristictum Centrolene heloderma Nymphargus grandisonae Nymphargus griffithsi Nymphargus lasgralarias
Ballux Creek
(2150–2200 m) Five Frog Creek (2100– 2150 m)
Heloderma Creek (2175–
2225 m)
Hercules Creek (2150–2200
m)
Chalguayacu River (1900–
1950 m)
Kathy´s Creek (1950–2050 m) Lucy´s Creek
(1825–1875 m)
Santa Rosa River (1800–
1875 m)
Ecology and natural history. Nymphargus lasgralarias sp. nov. inhabits small sized permanent streams (ca. 3 m width) within primary montane forest with minimal disturbance. The species is active during the night and emits advertisement calls from the tops of small sized ferns, small leaves, and long palm leaves 1–6 m above the stream ( Fig. 13 View FIGURE 13 D). Nymphargus lasgralarias sp. nov. occurs sympatrically with the following members of Centrolenidae : Centrolene ballux , Centrolene heloderma , Centrolene lynchi ( Duellman 1980) , Centrolene peristictum ( Lynch & Duellman 1973) , Nymphargus grandisonae , and Nymphargus griffithsi ( Table 3). Other anuran species sympatric along the creeks include: Hyloscirtus alytolylax ( Duellman 1972) , Pristimantis eugeniae ( Lynch & Duellman 1997) , Pristimantis calcarulatus ( Lynch 1976) , Pristimantis parvillus ( Lynch 1976) , and Pristimantis wnigrum ( Boettger 1892) .
Eggs are deposited on the tips of leaves over the stream and later expand into a hanging gelatinous mass upon absorption of water. We observed 12– 36 eggs per mass (mean = 25.4 ± 6.0 eggs; n = 23) for N. lasgralarias sp. nov. ( Fig. 13 View FIGURE 13 A–13B); we observed a single mass of N. griffithsi eggs containing 14 eggs ( Fig. 13 View FIGURE 13 C). The quantity of eggs per mass appears to be highly variable. The egg masses were distinguished by continual monitoring of calling male activity and observed close proximity of calling males. Nymphargus lasgralarias sp. nov. marks the third species of glassfrog in Ecuador with this egg habit type (i.e., eggs dangling from the tips of leaves) — after N. griffithsi and N. wileyi ( Guayasamin et al. 2006) .
The Saloya River basin is the type locality for N. griffithsi ( Goin 1961) , a locality that is about 11 km from the population of N. griffithsi at Reserva Las Gralarias ( Fig. 2 View FIGURE 2 ). These populations are nearly connected through the regional river system — the Canchupi River and Saloya River both flow into the Mindo River connecting the two systems, with ca. a 1 km gap between the start of the Canchupi River and “Five Frog Creek”. It is unknown whether N. griffithsi and N. lasgralarias sp. nov. populations occur in between these two observed localities. At Reserva Las Gralarias, N. griffithsi was observed to occur sympatrically with N. lasgralarias sp. nov. at “Five Frog Creek”, “Hercules Giant Tree Frog Creek”, and “ Heloderma Creek”. Another conspicuous spatial element is the absence of N. griffithsi and presence of N. lasgralarias sp. nov. at “Kathy’s Creek”, “Lucy’s Creek”, and the Chalguayacu Grande River, separated from the Santa Rosa River and associated creeks by less than 1 km ( Fig. 2 View FIGURE 2 ). See Figure 2 View FIGURE 2 for a map of the localities and Table 3 for a detailed species account for each creek.
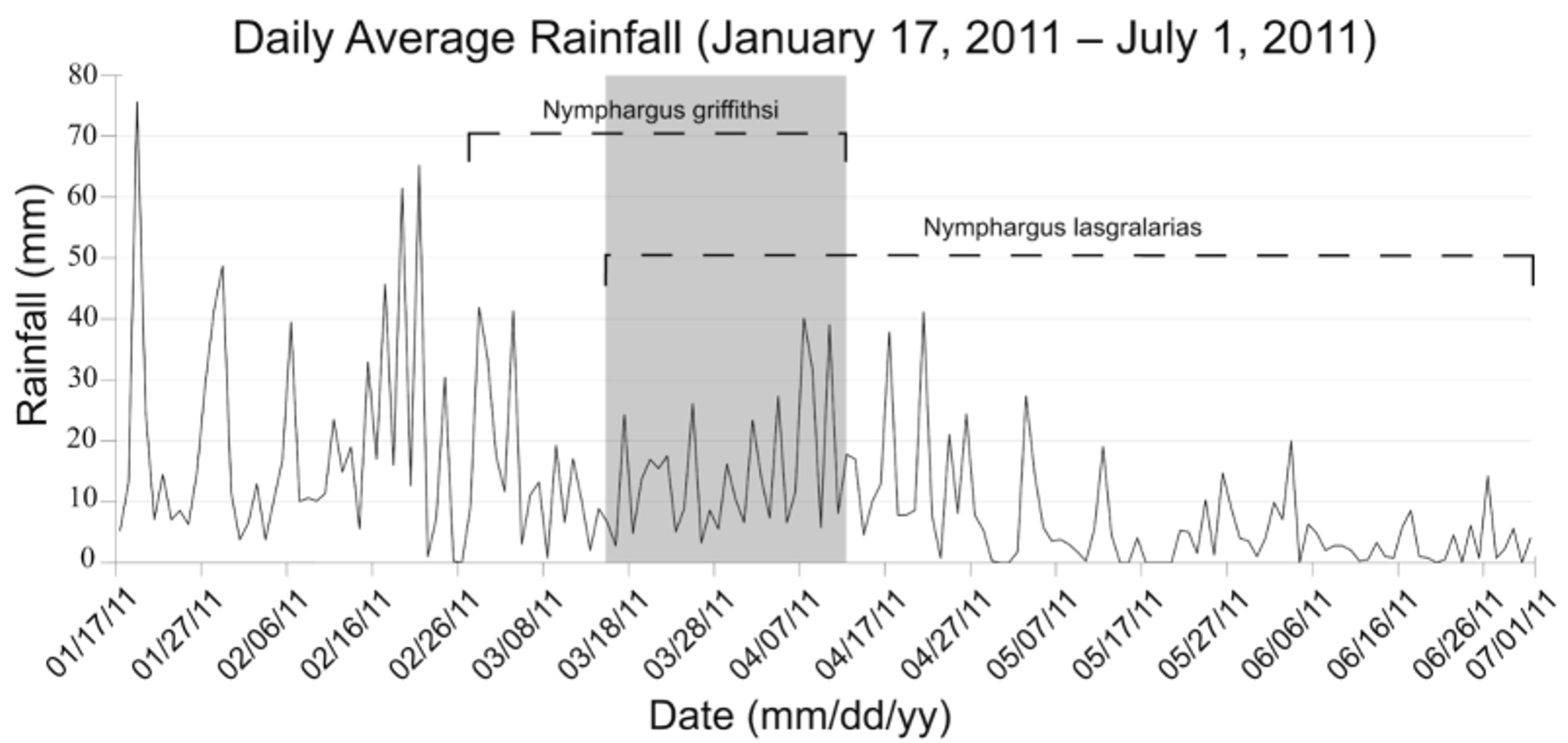
Nymphargus griffithsi and N. lasgralarias sp. nov. differ in timing of reproduction. We observed reproductive activity for N. lasgralarias sp. nov. between 5 April 2011 and 1 July 2011. The streams in which this species is present were surveyed several months prior to 5 April 2011, and no calling individuals were observed until that date. Calling activity was initially low, but increased dramatically at the end of April and peaked in the middle of May 2011, and slowly fell to a low density of calling males until final observations on 1 July 2011. We observed reproductive activity for N. griffithsi from 27 February to 14 April 2011 with the number of calling males remaining low. While these species occur sympatrically, they were never observed to be calling near each other or even in the same stream on the same date. In the sympatric creeks, N. griffithsi remained active until 14 April 2011 while N. lasgralarias sp. nov. was never observed in active advertisement calling with N. griffithsi in these creeks before this date. However, N. lasgralarias sp. nov. was observed advertisement calling beginning on 5 April 2011 — only in the creeks it occurred exclusively ( Table 3). After 14 May 2011, N. griffithsi ceased advertisement-calling activity and several days later, male N. lasgralarias sp. nov. began calling activity while N. griffithsi never resumed. Furthermore, the levels of precipitation during reproductive activity of Nymphargus griffithsi and N. lasgralarias sp. nov showed a significant difference (T-test: p <0.001). Nymphargus griffithsi is reproductively active when precipitation is higher (27 February 2011 until 14 April 2011; 0.8–41.9 mm, mean = 14.9 mm, SD = 11.1 mm). Nymphargus lasgralarias sp. nov. was active from 17 March 2011 to 1 July 2011 (rainfall = 0–41.9 mm, mean = 8.5 mm, SD = 9.6 mm; Fig. 14 View FIGURE 14 ). Although we suggest differential reproductive timing, we simply may not have observed simultaneous reproductive activity in these or other localities.
Combat behavior. Combat behavior has previously been reported by Duellman and Savitzky (1974) for Nymphargus griffithsi . They observed the behavior in a stream 9 km SE of Tandayapa, Pichincha. With the information at hand, it seems that their observations correspond to N. lasgralarias sp. nov., as they described the advertisement call as a “high pitched peep”, occurring in a series of two calls. Additionally, the illustration in the account does not indicate the presence of dark spots on the dorsum.
Etymology. The specific epithet lasgralarias is a noun in apposition and refers to the type locality of the new species, Reserva Las Gralarias (http://www.reservalasgralarias.com). We take pleasure in dedicating this species to the reserve and the team of people, led by Dr. Jane Lyons, for efforts on the conservation of Ecuadorian cloud forests and their commitment and support for research. As the English common name for this species, we suggest Las Gralarias Glassfrog. Moreover, we suggest Rana de cristal de Las Gralarias as the common name in Spanish.
TABLE 1. Morphometrics (in mm) of the holotype (MZUTI 096) and paratypes of Nymphargus lasgralarias sp nov.
| MZUTI 0 96 | MZUTI 0 94 | MZUTI 0 95 | MZUTI 0 93 | MZUTI 0 92 | MZUTI 0 91 | MZUTI 0 97 | |
|---|---|---|---|---|---|---|---|
| SVL Tibia length | 25.5 14.4 | 26.1 14.8 | 24.9 14.8 | 24.6 14.4 | 26.5 15.4 | 25.2 15.0 | 24.6 15.0 |
| Foot length Head length | 12.0 8.2 | 12.6 8.5 | 12.2 8.0 | 12.6 7.9 | 12.7 8.7 | 12.6 8.2 | 12.5 7.8 |
| Head width IOD Upper eyelid width Internarial distance | 8.7 2.4 2.4 2.1 | 8.8 2.6 2.4 2.3 | 8.7 2.5 2.2 2.2 | 8.6 2.4 2.1 2.0 | 9.0 2.8 2.4 2.3 | 8.4 2.7 1.9 2.2 | 8.6 2.3 2.4 2.3 |
| Eye diameter Tympanum diameter | 3.3 0.8 | 3.5 0.9 | 3.2 0.9 | 3.2 0.7 | 3.7 0.9 | 3.1 0.8 | 3.1 0.7 |
| Radioulna Hand length Finger I length Finger II length Disc of Finger III width | 5.9. 7.8 5.1 5.5 1.9 | 6.1 9.0 5.5 6.1 1.8 | 5.7 8.2 5.1 5.5 1.8 | 5.8 8.2 5.1 5.4 2.0 | 6.2 9.3 5.4 6.0 2.0 | 5.8 9.0 4.9 5.3 1.9 | 6.1 9.0 5.7 6.0 1.8 |
TABLE 2. A comparison of vocalization measurements between Nymphargus griffithsi and Nymphargus lasgralarias sp. nov.
| Call repetition rate (per minute) | 2.0–9.9 | 5.5 ± 2.7 | 1.1–1.9 | 1.6 ± 0.4 |
|---|---|---|---|---|
| Call duration (s) | 0.016–0.044 | 0.026 ± 0.006 | 0.103–0.148 | 0.122 ± 0.009 |
| Notes per call | 1 | – | 1 | – |
| Pulses per call | 1–3 | 1.5 ± 0.6 | Tonal–3 | 2.4 ± 0.5 |
| Most frequent (pulses per call) | 1 | – | Tonal | – |
| Call rise time (s) | 0.002–0.013 | 0.007 ± 0.003 | 0.013–0.104 | 0.066 ± 0.020 |
| Dominant frequency (Hz) | 3445.3–3962.2 | 3691.4 ± 131.9 | 3789.8–4306.6 | 4107.4 ± 105.5 |
| Frequency modulation (Hz) | -172.3–172.3 | 21.7 ± 69.3 | 0–344.6 | 199.3 ± 116.2 |
| Initial frequency of the fundamental frequency (Hz) | 2561.0–3441.0 | 3063.6 ± 162.5 | 2272.0–3777.0 | 3290.6 ± 225.1 |
| Lower frequency of the fundamental frequency (Hz) | 2939.4–4145.2 | 3236.3 ± 168.7 | 3400.4–3826.3 | 3672.3 ± 115.3 |
| Upper frequency of the fundamental frequency (Hz) | 3887.7–4473.4 | 4139.8 ± 139.7 | 4160.2–4702.2 | 4520.5 ± 131.3 |
| 1st harmonic (Hz) | 6546.1–8096.5 | 7298.9 ± 305.8 | 6546.1–8613.3 | 8071.8 ± 268.9 |
| 2nd harmonic (Hz) 3rd harmonic (Hz) | 9991.4–12058.6 13781.2–14928.0 | 11034.2 ± 478.3 14317.1 ± 245.5 | 7579.7–15503.9 16020.7–17054.3 | 12673.8 ± 780.3 16437.0 ± 232.5 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |