Aceria argentae, Pye, Daniel R. L., 2011
|
publication ID |
https://doi.org/10.5281/zenodo.207837 |
|
DOI |
https://doi.org/10.5281/zenodo.6182978 |
|
persistent identifier |
https://treatment.plazi.org/id/03EB87BF-6273-3213-5AB5-A53FEB95F892 |
|
treatment provided by |
Plazi |
|
scientific name |
Aceria argentae |
| status |
sp. nov. |
Aceria argentae n. sp.
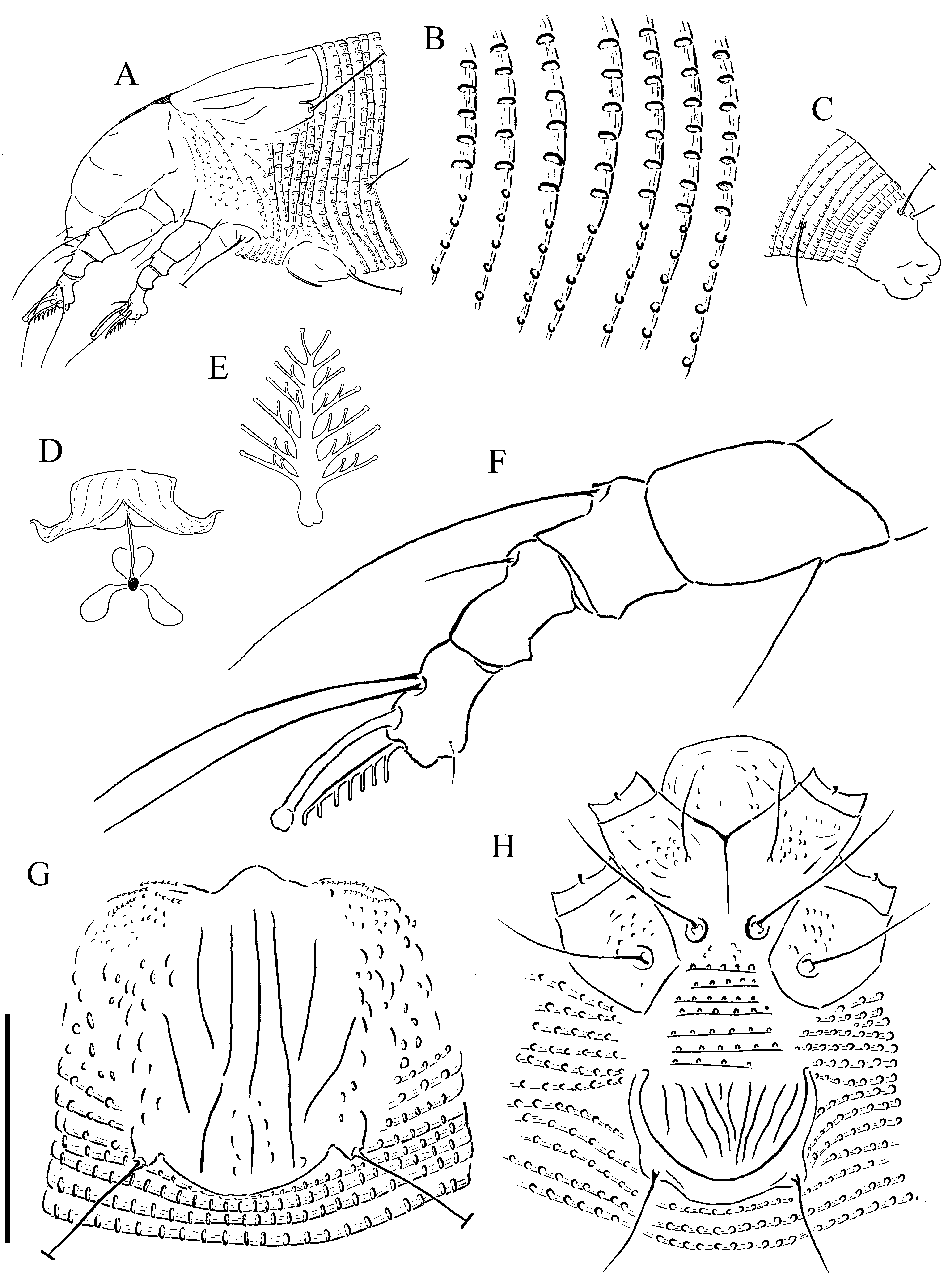
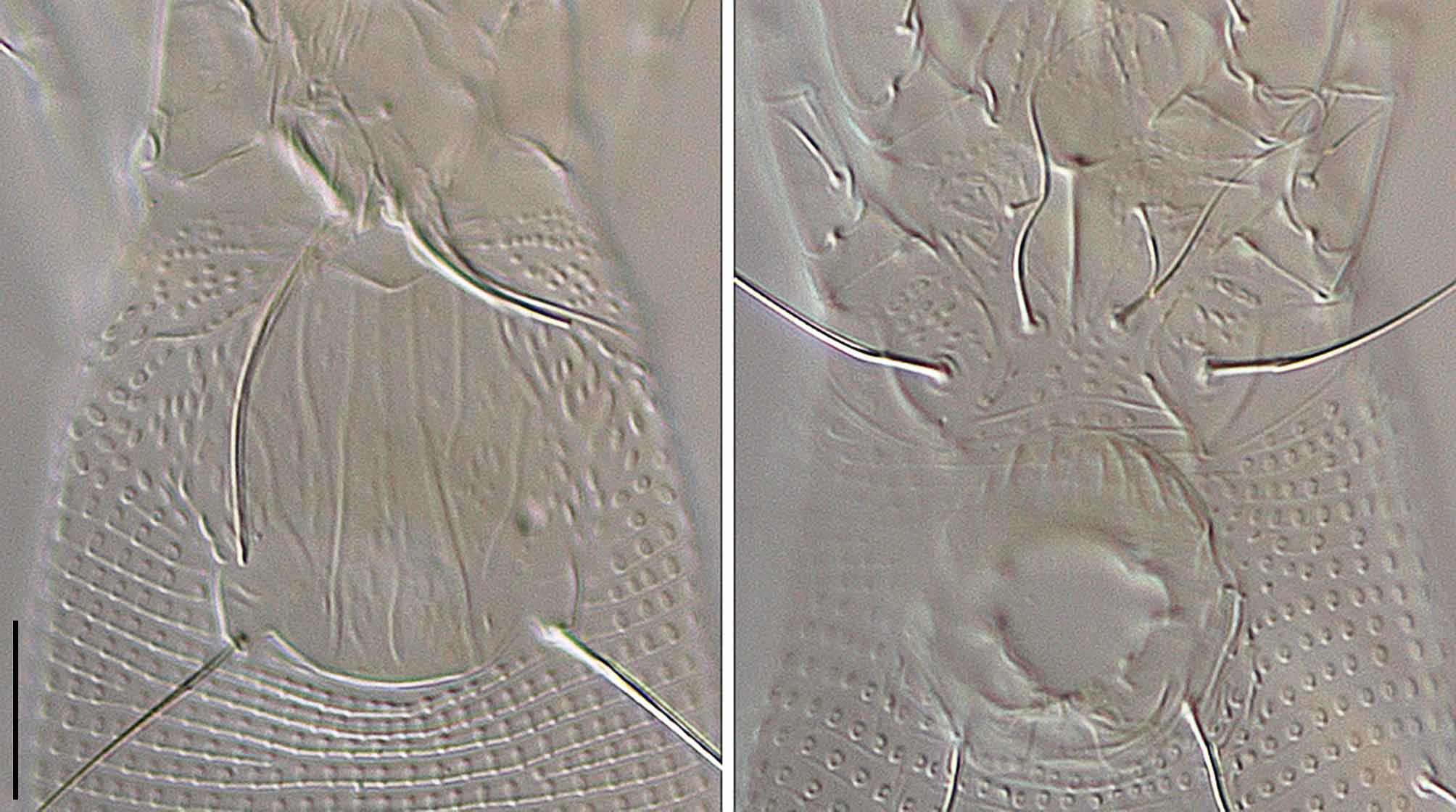
( Figs 1 View FIGURE 1 & 2 View FIGURE 2 )
FEMALE (n = 7). Body vermiform, 168 (149–176), 43 (37–43) wide. Gnathosoma projecting forward and down, chelicerae 23 (23–26, n = 4), setae d 4 (4, n = 4), subcapitulum ornamented with short lines and dashes. Prodorsal shield 25 (24–27), 31 (26–31) wide. Prodorsal shield ornamented with a pattern of longitudinal lines, with longitudinal microtubercles laterally. Median line nearly complete, broken at posterior; admedian lines usually complete, rarely broken. Submedian lines converge toward the admedian lines at about half way. Lateral margins of the prodorsal shield with elongate microtubercles similar to the microtubercles on opisthosomal annuli. Prodorsal shield lobe small and apically rounded. Tubercles sc 18 (16–18) apart, setae sc 24 (21–25, n = 6). Leg I 28 (27–28); femur 8 (8–10), seta bv 9 (8–10, n = 6); genu 5 (4–5), seta l ʺ 18 (16–20); tibia 7 (6–7), seta l ʹ 7 (5–7); tarsus 6 (6), seta ft ʹ 18 (15–19), seta ft ʺ 18 (17–19, n = 6); seta u ʹ 2 (2–3, n = 3); solenidion 8 (7–8), expanded distally; empodium 7 (6–7), 7-rayed. Leg II 24 (22–26); femur 8 (7–8), seta bv 7 (6–7); genu 4 (3–4), seta l ʺ 8 (5–8, n = 6); tibia 5 (4–5); tarsus 6 (5–6), seta ft ʹ 3 (3–5), seta ft ʺ 21 (18–21); seta u ʹ 2 (2, n = 2); solenidion 9 (7–9), expanded distally; empodium 7 (6–9), 7-rayed. Coxae I and II ornamented with short lines and sub spherical granules on anterior part, with posterior parts smooth and unornamented. Setae 1b 6 (6–8, n = 4), 7 (7) apart; setae 1a 20 (19–20, n = 4), 5 (5–6) apart; setae 2a 38 (30–38), 15 (15) apart; tubercles 1b and 1a 7 (6–7) apart; tubercles 1a and 2a 6 (6) apart. Coxigenital region with 7 (7, n = 2) annuli. Opisthosoma with 70 (61–70) dorsal annuli, 58 (57–65) ventral annuli. Annuli completely microtuberculate. Dorsal microtubercles longitudinally elongate on posterior margin of annuli ( Fig 1 View FIGURE 1 . B). Ventral microtubercles smaller, more rounded ( Fig 1 View FIGURE 1 . B). Setae c2 13 (11–13), on annulus 4 (4– 5), 38 (36–38) apart; setae d (42–50, n = 5), on annulus 15 (14–17), 32 (31–32) apart; setae e 13 (10–14), on annulus 30 (29–35), 18 (17–18) apart; setae f 16 (14–19), on annulus 52 (51–59), 11 (10–11) apart, 7 (6–7) from rear. Last 5 (4–6) annuli with ventral elongate linear microtubercles. Dorsal posterior annuli with sparse, reduced microtubercles, last 2 (2–4) annuli with elongate linear microtubercles. Setae h2 (63–69), 9 (8–9) apart; setae h1 4 (3–4), 5 (4–5) apart; h2 and h1 tubercles 2 (2–3, n = 3) apart. Genital coverflap ornamented with 10 (9–10) longitudinal lines, 11 (11), 18 (17–18) wide. Setae 3a 17 (11–17), 13 (11–13) apart. Internal genital apodeme extended forward.
MALE (n = 2) Similar to female. Very few male specimens were found. A full description was not possible because of the mounting position (lateral) of specimens and extensive damage to morphological characters. Body vermiform, 150–168. Gnathosoma projecting forward and down. Setae sc 21–23. Leg I 27–28, empodium 6 or 7- rayed. Leg II 23, empodium 6 or 7-rayed. Opisthosoma with 63 dorsal annuli, 55 ventral annuli. Annuli completely microtuberculate, microtubercles same as female. Setae c 2 12–13, on annulus 4; setae d 38–43, on annulus 13–14; setae e 11–12), on annulus 27; setae f 13–15, on annulus 49–50, 5–6 from rear. Last 5 (4–6) annuli with ventral elongate linear microtubercles. Setae h2 50–56; setae h1 4. Setae 3a 9–10.
NYMPH. (n = 3) Body vermiform, 141–154. Gnathosoma projecting forward and down. Setae sc 18–20. Leg I 21–22, empodium 6-rayed. Leg II 17–20, empodium 5 or 6-rayed. Opisthosoma with 62–64 dorsal annuli. Annuli completely microtuberculate, microtubercles subsperical on dorsal and ventral annuli. Setae c 2 9–10, setae d 26–32, setae e 7–8, setae f 11, 5–6 from rear. Last 5 (4–6) annuli with ventral elongate linear microtubercles. Setae h2 43–47, setae h1 3. Setae 3a 7.
Host plant. Leucadendron argenteum (L.) R. Br. ( Proteaceae ), native to South Africa. The International Union for Conservation of Nature (IUCN) lists L. argenteum as “Vulnerable” on the IUCN Red List ( Hilton-Taylor et. al 1998).
Relation to the host. The mites were found vagrant and feeding around the new growth and flower head. No host symptoms were observed.
Collection details and type locality. The plant material was intercepted by Maureen Tierney (PHSI) at Heathrow Airport, Middlesex, England, 13.v.2009; originally imported from Kirstenbosch Botanical Gardens, Cape Town, South Africa.
Type material. Holotype female on a microscope slide with an additional paratype female on a separate slide deposited at the ARC-Plant Protection Research Institute, Pretoria, South Africa (via Charnie Craemer). Four microscope slides, each with one paratype female; one microscope slide with a single paratype female and four immature stages; one male paratype on a slide, and two slides, each with one paratype male along with 4 immatures deposited in the collections at Fera (reference number 20908599).
Etymology. The specific name, argentae (latin feminine), is derived from argenteum , the specific name of the host plant.
Discussion. Aceria argentae is the first record of an eriophyoid mite inhabiting L. argenteum . There are nine other species of eriophyoids known to inhabit plants from the family Proteaceae . Aceria proteae Meyer 1981 was first described from Protea repens (L.) L. and later recorded from Leucadendron sp. R. Br., Leucospermum cordifolium (Salisb. ex Knight) , Leucospermum cuneiforme (Burme. F.) , Leucospermum linifolium (Jacq.) R. Br. , Mimetes cucullatus (L.) and many other Protea species ( Meyer 1996). This species is also recorded as the vector of an organism that causes witches’ brooms on many plant species in the Proteaceae ( Meyer 1981; Coetzee, Rust & Latsky 1986; Meyer 1996). Aceria rusti Meyer & Ueckermann 1992 and Eriophyes meyerae Ueckermann 1993 were both originally described from Brabejum stellatifolium L. whereby A. rusti causes galls on the upper and lower leaf surfaces with E. meyerae being found in association; Calepitrimerus heliciopus Chandrapatya & Boczek 2000 on Heliopsis terminalis (Kurz.) Sleumer and Cosella formosana Huang & Wang 2003 , Asetacus obscurus Huang & Wang 2009 and Taicolopodacus primus Huang & Wang 2009 , all leaf vagrants on Helicia formosana Hemsl. described from Taiwan. Diptilomiopus davisi Keifer 1969 , another leaf vagrant, was originally described from Australia on Macadamia tetraphylla L. A. S. Johnson. Finally , an Aculus sp. was found on an unidentified host plant in the Proteaceae , but no further details were given ( Coetzee, Rust & Latsky 1986). Of these nine species, A. proteae is known to be the most economically damaging, and can cause serious losses to plantings of ornamental proteas ( Meyer 1996).
Differential diagnosis. When compared to the other eriophyoid mite species found on plants in the family Proteaceae , A. argentae is most similar to A. proteae [based on the original description and illustrations ( Meyer 1981)] but differs by having: 7-rayed empodia (6-rayed in A. proteae ); the absence of a dart-shaped mark at the posterior of the median line on the prodorsal shield (present in A. proteae ); submedian lines on the prodorsal shield that converge toward the admedian lines (the first submedian line diverges onto the second submedian at about halfway on A. proteae ); scapular setae that are about 21–25µm long (about 55µm long on A. proteae ).
Attempts were made to examine the type slides of A. proteae but unfortunately the slides had deteriorated to a point where they could not be used for morphological studies and were subsequently disposed of many years ago (C. Craemer & E. A. Ueckermann, personal communications, 2011). In addition, no other specimens of A. proteae from the type plant hosts are currently available for study in the Mite Collection of the National Collection of Arachnida, Pretoria, South Africa. There appears to be a complex of Aceria species which causes witches' brooms on different Protea species and further work is required to clarify the taxonomy of this group (C. Craemer, personal communication, 2011).
Dichotomous key to the species of eriophyoid mites hitherto found on plants in the Proteaceae
1. Gnathosoma small; empodia entire............................................................................ 3
- Gnathosoma large; empodia entire or deeply divided.............................................................. 2
2. Empodia entire.............................................................................. Asetacus obscurus
- Empodia deeply divided..................................................................... Diptilomiopus davisi
3. Legs with tibia and tarsi fused................................................................................ 4
- Legs with tibia and tarsi separate.............................................................................. 5
4. Coxal setae 1b absent........................................................................ Cosella formosana
- Coxal setae 1b present..................................................................... Taicolopodacus primus
5. Scapular setae situated ahead of the posterior margin of the prodorsal shield, with plicate tubercles directing setae centrad....... 6
- Scapular setae situated at the posterior margin of the prodorsal shield, directing setae backwards............................ 7
6. Opisthosoma more fusiform, with a median ridge........................................... Calepitrimerus heliciopsus
- Opisthosoma vermiform, annuli subequal, with no ridges............................................ Eriophyes meyerae
7. Opisthosoma more fusiform, ventral annuli more numerous than dorsal............................................................................................... Aculus sp. (recorded by Coetzee et al. 1986, species not identified)
- Opisthosoma vermiform, annuli subequal....................................................................... 8
8. Coxal plates unornamented, smooth; scapular setae about 9–12 µm long; empodia 5-rayed...................... Aceria rusti
- Coxal plates ornamented with granules or lines; empodia 6 or 7-rayed................................................ 9
9. Dart shaped mark present at the posterior of the median line on the prodorsal shield; scapular setae about 55µm long; empodia 6-rayed................................................................................... Aceria proteae
- Dart shaped mark absent at the posterior of the median line on the prodorsal shield; scapular setae about 21–25µm long; empodia 7-rayed............................................................................. Aceria argentae n. sp.
Additional remarks. Due to the increase in international plant trade (Levine & D'Antonio 2003), there has been a rise in the number of non-native eriophyoid mite interceptions in the United Kingdom. Furthermore, the changes in climatic conditions in recent decades may also provide more favourable environments for non-native pests to spread and breed ( Burgiel & Muir 2010). As shown in this study, where a sample of L. argenteum View in CoL was originally collected due to the presence of a leaf mining moth, eriophyoid mites can often be overlooked. This is probably because of their small size which makes initial detection difficult, giving eriophyoid mite species a high potential to become adventive, an issue discussed by Navia et al. (2010). Recent examples from the UK include incursions of Aceria kuko (Kishida 1927) causing galls on Lycium View in CoL sp. ( Solanaceae View in CoL ) (Goji berry) from China, via The Netherlands (Ostojá-Starzewski 2009), and numerous findings of Aculops fuchsiae Keifer 1972 ( fuchsia View in CoL gall mite) (Ostojá-Starzewski et al. 2009), which is native to South America and causes serious damage to Fuchsia View in CoL spp. ( Onagraceae View in CoL ). There is currently little data on any potential economic impact of A. kuko in the UK, whereas A. fuchsiae , a European and Mediterranean Plant Protection Organization (EPPO) and European Union (EU) quarantine listed pest, is spreading and becoming an increasing problem in southern England (Fera, unpublished geographical records, 2011).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Eriophyoidea |
|
Family |
|
|
Genus |