Acanthoniscus richardsonae, Rodríguez-Cabrera & de Armas, 2023
|
publication ID |
https://doi.org/10.1590/2358-2936e2023006 |
|
publication LSID |
lsid:zoobank.org:pub:4970E3C2-A1DB-4AAE8961-FBAA26505ED4AB |
|
DOI |
https://doi.org/10.5281/zenodo.10926653 |
|
persistent identifier |
https://treatment.plazi.org/id/03E987EB-FF0F-FFFB-FEBE-FCAEFB2FFD52 |
|
treatment provided by |
Felipe |
|
scientific name |
Acanthoniscus richardsonae |
| status |
sp. nov. |
Acanthoniscus richardsonae sp. nov.
( Figs. 1 View Figure 1 , 3–8 View Figure 3 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 , 10 View Figure 10 )
ZooBank: urn:lsid:zoobank.org:act:
Acanthoniscus spiniger (partim): Richardson, 1909: 431–434 (misidentification). — Budde-Lund, 1910: 11 (partim). — Richardson, 1910: 495 (partim). — Arcangeli, 1927:135 (partim). — Van Name, 1936: 402–403 (partim). — Schmalfuss, 2003: 4 (partim). — Schmidt and Leistikow, 2004: 4 (partim). — Jass and Klausmeier, 2006: 4, 6, 25 (partim).
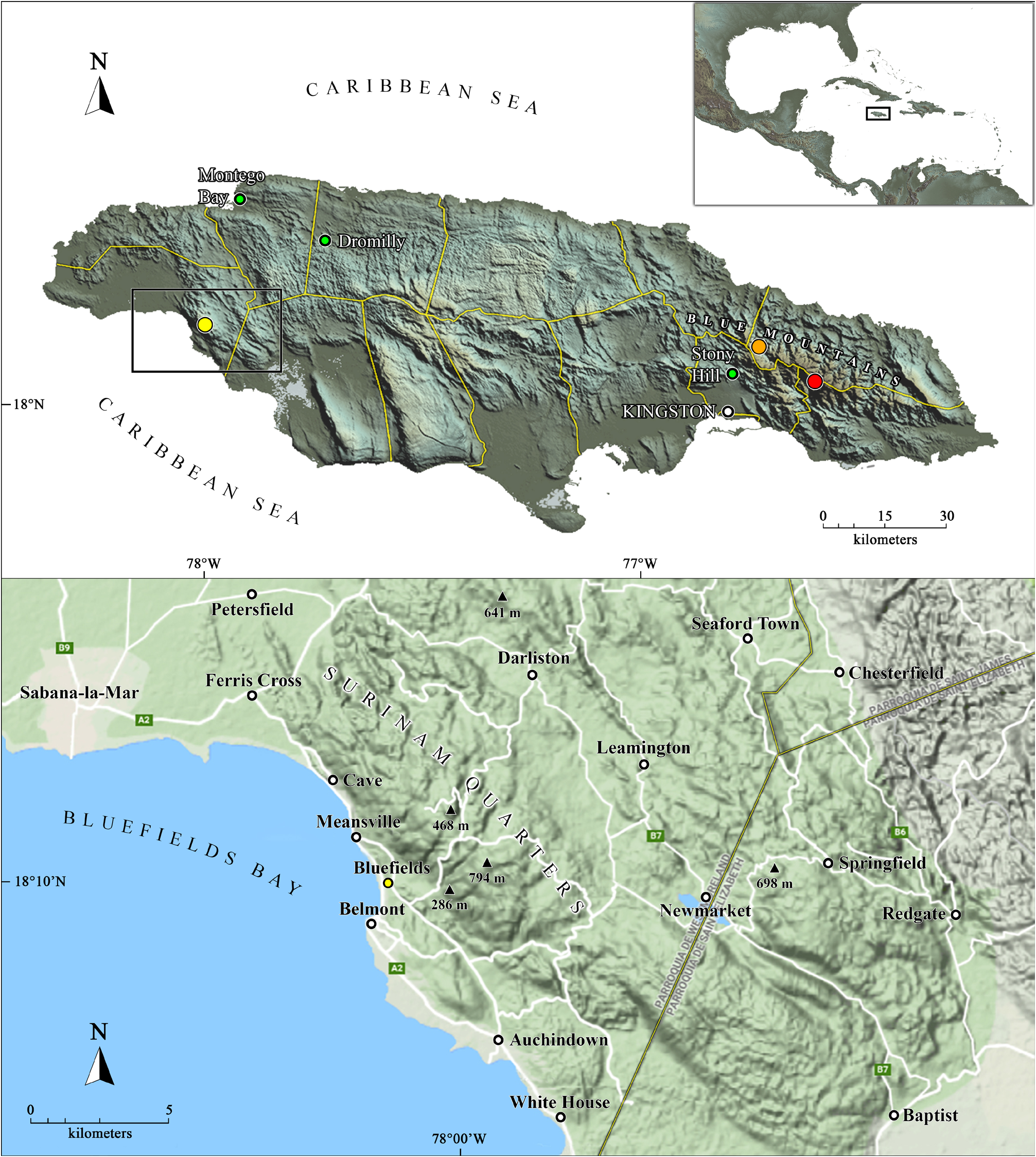
Type material. Holotype: 1 ♂ ( CZACC 5.0500 View Materials ), around the ranger station ( 18°03’12.6”N 76°33’47.2”W, approximately 1,250 m a.s.l.), Blue Mountains , Saint Thomas Parish, Jamaica ( Fig. 1 View Figure 1 ), 15 November 2013, Abel Pérez-González and Franklyn Cala Riquelme (collectors’ code: “JA 10-2”, “1671Caribbean”) GoogleMaps . Paratypes: 2 ♂♂, 6 ♀♀, 2 juvs. ( CZACC 5.0501 View Materials ), same data as the holotype GoogleMaps .
Additional material (not paratype). Jamaica (unknown locality), a single specimen collected by Hubbard in 1877, the original label reads: “ Oniscus spiniger . Jamaica ” ( Richardson, 1909: 431). The current catalog number is: USNM 41501 (https://n 2t.net/ark:/65665/3ac606ed9-99f9-4214- 891d-1e92ed7ccbe4). This specimen served as the basis for Richardson’s (1909) description, assumed as a redescription of A. spiniger . It was not possible for us to review this specimen directly or by pictures.
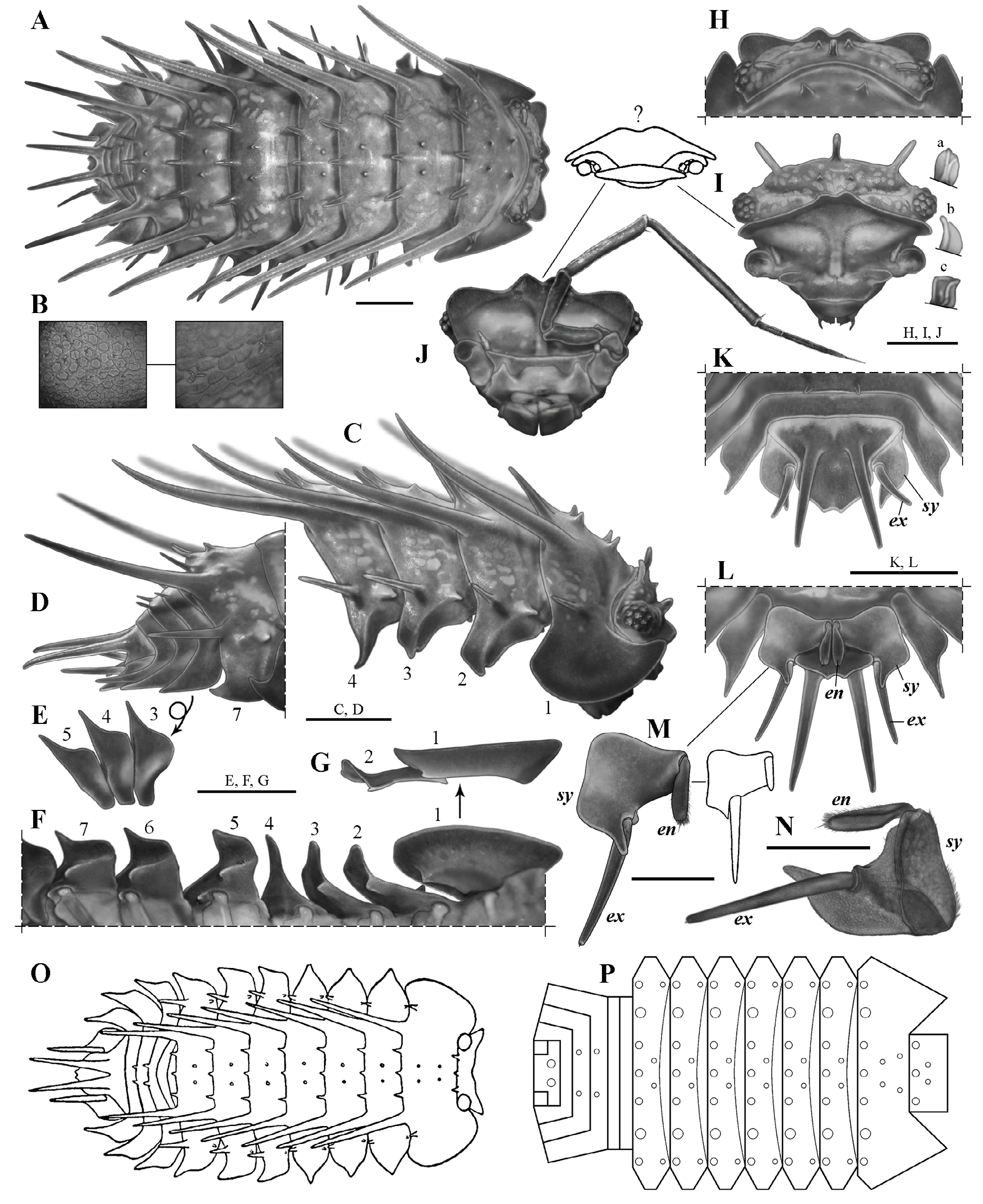
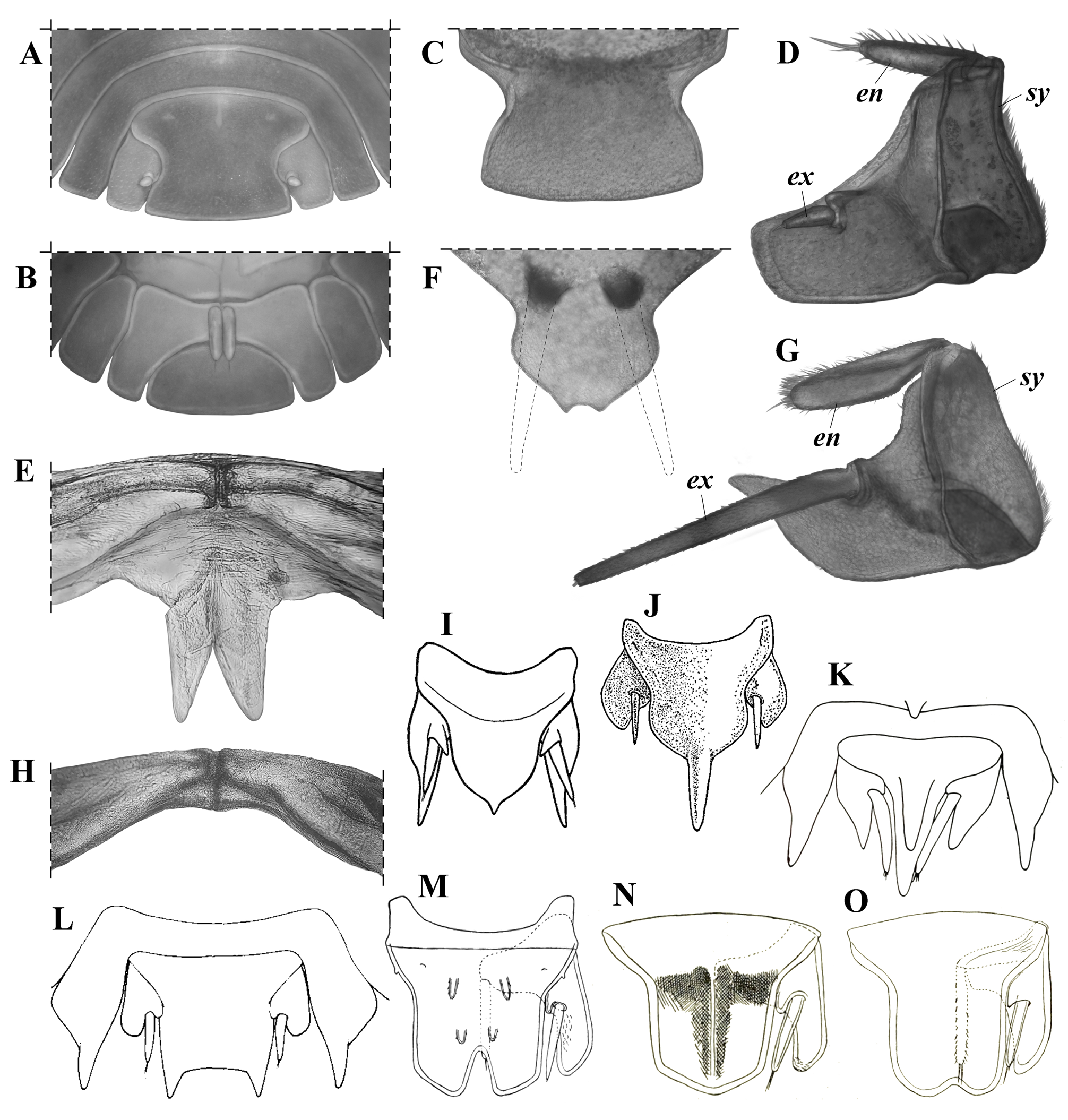
Diagnosis (Diagnosis and description are the same for the holotype and the paratypes, unless specified). Maximum total length: 7.2 mm in males ( holotype) and 8.5 mm in females (see Variation). Cephalothorax with rounded lateral lobes, and a well-developed, deeply notched median lobe, forming two triangular projections, dorsal surface with a pair of small paramedian spiniform tubercles and three medium-sized, club-like, posteromedian spines ( Fig. 4C, H–J View Figure 4 ). Pereonite 1 with two pairs of paramedian spiniform tubercles and three medium-sized posteromedian spines; pereonites 2–7 with a pair of paramedian spiniform tubercles and three medium-sized posteromedian spines ( Fig. 4A, C–D, O–P View Figure 4 ); epimera more or less quadrangular (except epimera 4: subtriangular, very narrow and sharp-pointed), with anterior (epimera 2–3) or posterior (epimera 5–7) margins widely convex and produced (forming an almost square angle) ( Fig. 4C, F View Figure 4 ); coxal plates 5–7 with a medium-sized tooth-like process, in medial position, forming a schisma and extending anteriorly into an oblique, very shallow, longitudinal groove, and with a very small submarginal tooth-like process, in basal position, just posterior to pereiopod’s junction, forming a second very small schisma; latter two schisma forming a continuum engaging with the contiguous posterior pereon epimera during conglobation ( Fig. 4F View Figure 4 ). Pleonites 3–4 with a pair of small posteromedian spines; pleon epimera 3–5 with anterior margin widely convex and produced (forming an almost square angle) ( Fig. 4E View Figure 4 ). Pleotelson with posterior margin ending in two small triangular projections separated by a shallow notch ( Figs. 4D, K–L View Figure 4 , 8F View Figure 8 ). Uropod sympodite “subtrapezoidal,” outer margin widely convex and produced (forming an almost square angle), medial-posterior angle narrow, and approximately at same level or slightly surpassing the distal margin of pleotelson; endopodite surpassing the insertion of exopodite, but not surpassing either the posterior margin of sympodite or the distal margin of pleotelson ( Figs. 4K–N View Figure 4 , 8G View Figure 8 ).
Cephalothorax ( Fig. 4C, H–J View Figure 4 ). Nearly three times (2.7) as wide as long, with rounded lateral lobes, and well-developed, deeply notched median lobe, forming 2 triangular projections slightly bent upward. Dorsal surface with pair of small paramedian spiniform tubercles and 3 medium-sized, club-like, posteromedian spines, one on the median line and one on either side close to the eyes; lateral spines about half length of cephalothorax, median spine smaller than lateral ones. Eyes with tiny scale-setae inserted on inter-ommatidia space (observable only at> 80×).
Pereon ( Figs.3 View Figure 3 , 4A,C–D, F–G, O–P View Figure 4 ). Pereonite 1, 3 times as wide and 1.5times as long as cephalothorax, with 2 very long, slightly sinuous dorsolateral spines (twice the length of pereonite1), 2 pairs of paramedian spiniform tubercles (2 on either side of median line), 3 medium-sized posteromedian spines (0.3 times as long as pereonite 1, 1 on the median line), and smaller lateral spine on either side near posterior margin, directed backward and slightly bent upward; pereonites 2–7 similar, four times as wide as long (pereonite 7 slightly narrower), with 2 very long slightly sinuous dorsolateral spines (twice the length of pereonite 1), pair of paramedian spiniform tubercles (one on either side of median line), 3 medium-sized posteromedian spines, smaller lateral spine on each side near posterior margin, straight and directed backward and slightly outward (gradually increasing in length posteriorly, being lateral spines of seventh tergite about half as long as dorsolateral spines), and spiniform tubercle near anterior margin and directed forward (engaging with previous tergite during conglobation, decreasing in size posteriorly, vestigial on pereonite 7). Epimera 1 with posterior angle and outer margin broadly rounded, anterior angle more acutely produced, dorsal surface concave; epimera 2–3 more or less quadrangular and narrow, with anterior margin concave and posterior margin widely convex, produced distally and then straight toward median region, outer angle acutely produced and directed forward; epimera 4 subtriangular, very narrow and sharp-pointed, and strongly differentiated from epimera 2–3; epimera 5–7 more or less quadrangular, with anterior margin widely convex and produced distally (forming almost square angle), posterior margin concave, and outer angle acutely produced (sharp-pointed) and directed backward. Coxal plates 1–3 with small inner tooth-like process near posterior margin and directed backward, in medial position, forming small schisma and, in coxal plate 1, extending into shallow transverse groove toward base of coxa; coxal plate 4 without tooth-like processes; coxal plates 5–7 with medium-sized tooth-like process, in medial position and near posterior margin, directed backward, forming schisma and extending anteriorly into oblique, very shallow, longitudinal groove, and with very small submarginal tooth-like process, in basal position, just posterior to pereiopod junction, forming second very small schisma; the latter 2 schisma forming continuum engaging with contiguous posterior pereon epimera during conglobation.
Pleon ( Fig. 4A, D–E View Figure 4 ). Tergites 1, 2, and 5 without spines; tergites 3–4 with pair of small posteromedian spines (one on either side of the median line) as long as pleonite length; epimera of pleonites 3–5 acutely produced, similar in shape and size, with anterior margin widely convex and produced medially(forming almost square angle), posterior margin straight, and outer angle acutely produced (sharp-pointed).
Pleotelson( Figs.4D, K–L View Figure 4 , 8F View Figure 8 ). Basal part 1.7 times wider than distal part, 1.4 times as wide as long, with both dorsal spines 1.3–1.5 times as long as pleotelson; distal margin ending in 2 small triangular projections separated by shallow notch (0.3 times as deep as the median diameter of pleotelson spines).
Uropods( Figs.4K–N View Figure 4 , 8G View Figure 8 ).Sympoditesubtrapezoidal, 1.3times as long as wide, ventral surface flat, outer margin widely convex and produced (forming almost square angle), medial-posterior angle narrow and approximately at same level, or slightly surpassing, distal margin of pleotelson, medial margin with very small inner lobe above and basal with respect to insertion of exopodite; endopodite surpassing insertion of exopodite, but not surpassing either posterior margin of sympodite or distal margin of pleotelson (not visible dorsally); exopodite 1.1 times as long as sympodite and surpassing posterior margin of sympodite by 60% of its length.
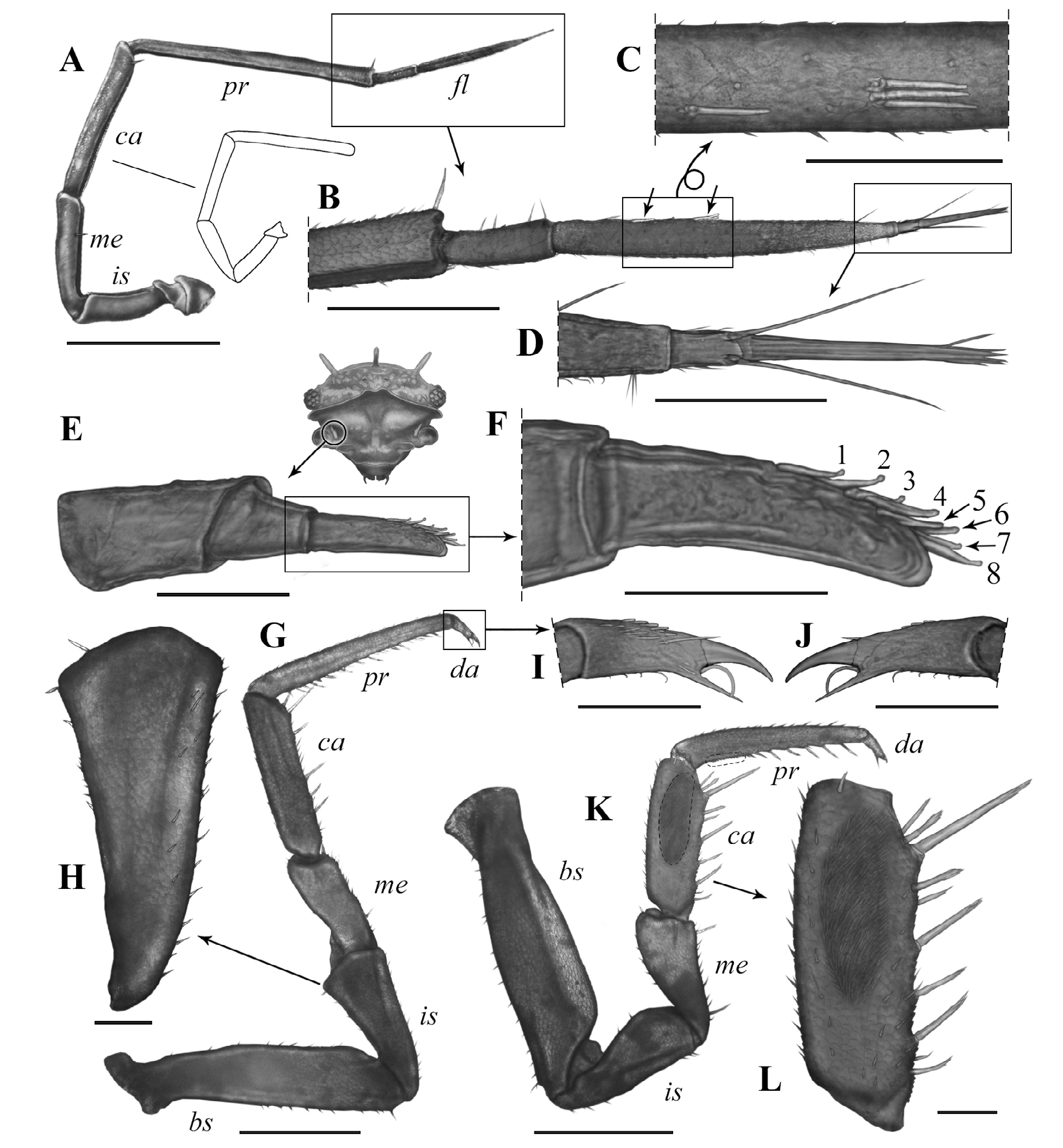
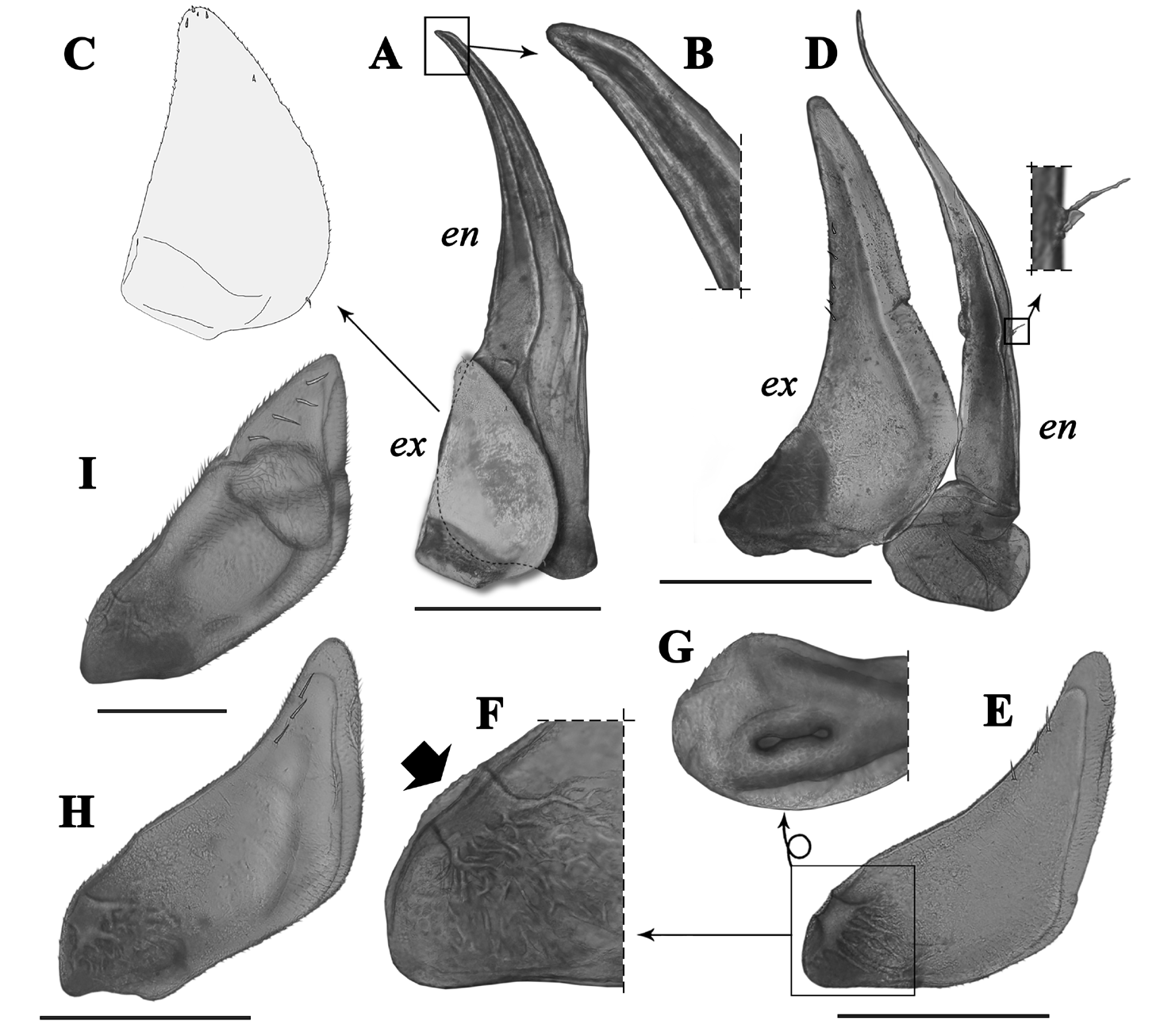
Antennae ( Figs.4J View Figure 4 , 5A–D View Figure 5 ). First antenna 3-jointed; first article thick, sub-cylindrical, twice the length of second article and 1.6 times as long as third article; second article shortest and sub-conical in shape; third article narrow and asymmetric, slightly curved, with row of eight aesthetascs in subapical position.Second antenna flagellum with distal article 3 times longer than basal article, with aesthetasc group on basal-most quarter and other near middle region (formula: 1–3), and tuft of 3 small setae near distal end, apical cone as long as basal article of flagellum and hence 3 times shorter than distal article, with 2 long lateral free sensilla inserted on basal-most quarter and nearly reaching tip of cone; ischium nearly as long as merus, carpus 1.3 times longer than merus, propodus 1.5 times longer than carpus and 1.3 times longer than flagellum; ischium, merus, carpus,and propodus with deep groove along entire inner surface.
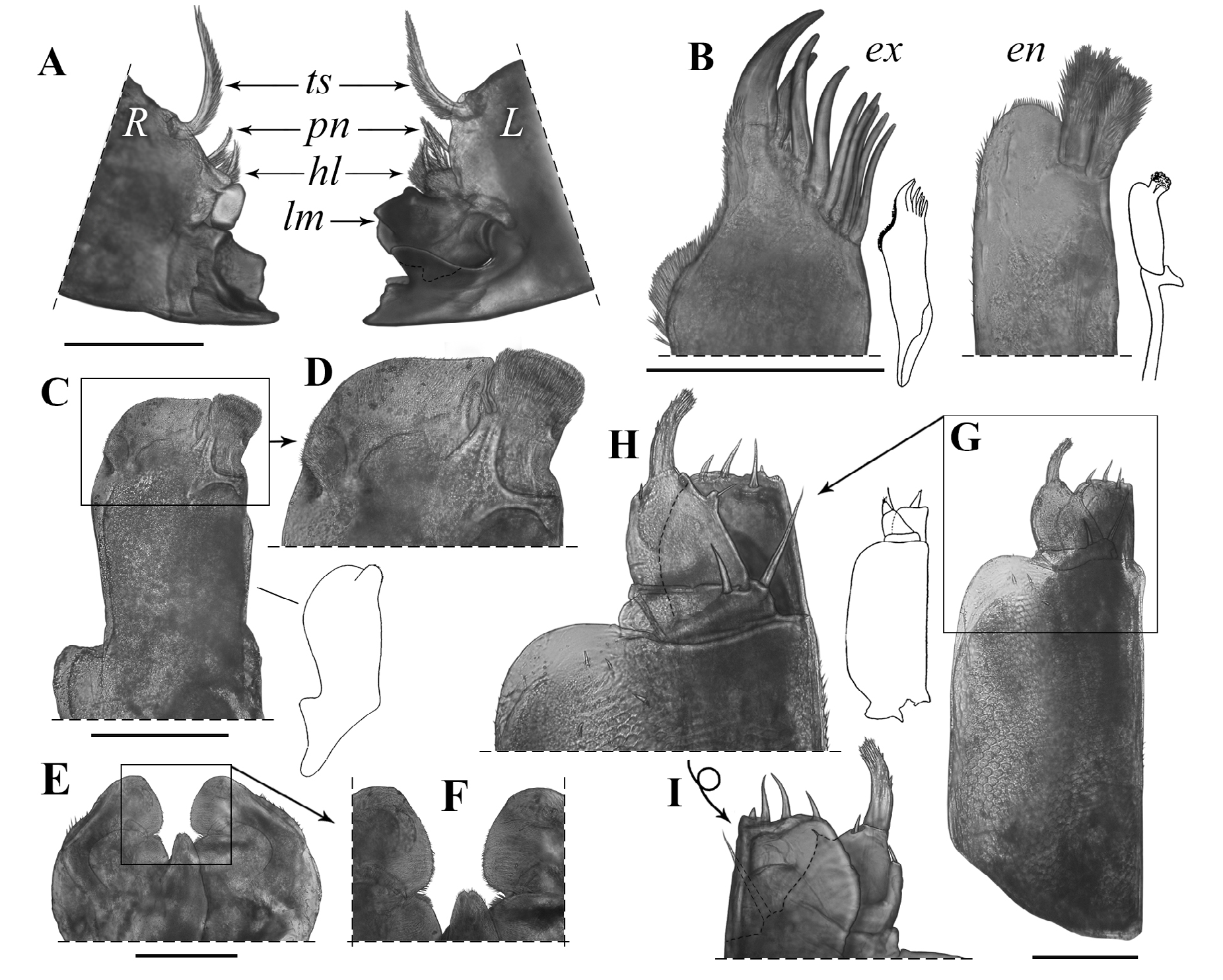
Mouthparts ( Fig.6 View Figure 6 ). Left mandible in anterior view (in distal-proximal order) with a narrow quadricuspid incisor process, lacinia mobilis bicuspid with smaller inner lobe, hairy lobe bearing 2 penicils, 1 free penicil, and tuft of hairy setae as molar process.Right mandible in anterior view (in distal-proximal order) with wider quadricuspid incisor process, oval hyaline process, hairy lobe bearing 2 penicils, and tuft of hairy setae as molar process. Hypopharynx with middle lobe notched at tip, with groups of relatively large setae along outer margin of lateral lobes, inner margin of lateral lobes densely hirsute. First maxilla outer endite with outer margin deeply curved distally and with marginal fringe of small setae and 10 simple tooth-like setae of approximately equal in length: outer group composed of 4 strong setae (lateral-most considerably stronger) and small triangular lobe, inner group composed of 6 slender setae, with very small subapical seta between both groups of tooth setae; inner endite with 2 subapical thickset penicils and slightly produced, rounded laterodistal corner. Second maxilla outer lobe wider and sensilla scattered, with outer notch; inner lobe narrower, with sensilla concentrated in dense field on mediodistal corner; with 2–3 differentiated setae in incision between both lobes, on medial margin of inner lobe. Maxilliped basipodite with nearly parallel outer and inner margins and wide lamella on distal portion of outer margin, with scaly surface and scattered small setae; endite apically truncate, with 3 enlarged subapical setae on posterior surface and outer corner with 2 small rounded lobes, one of them ending in tiny buttonlike seta; palpus with middle and distal article fused; basal article bearing 2 strong setae, outer one nearly reaching distal margin of endite; middle article with 2 “tufts” on medial margin bearing small single seta each (basal-most one on produced lobe), and single seta on outer-distal margin; apical article narrow and bent inward, ending in tuft of setae and with tiny subapical seta on outer margin.
Pereiopods ( Fig. 5G–L View Figure 5 ). Basis of pereiopod 1 with dorsal ridge and dorsal surface with longitudinal groove; carpus ventral surface with 9 multi-apex setae, with one in subapical position nearly duplicating size of others; cleaning apparatus, antennal brush, or antenna-grooming brush broad and covering slightly over half of carpus anterior surface and also present on ventral surface of basal-most third of propodus. Male pereiopod 7 without special modifications, with 6 multi-apex setae on ventral surface of carpus, one in subapical position duplicating size of the others; ischium sub-conical, 2.3 times as long as wide.
Pleopods ( Fig. 7 View Figure 7 ). Exopodites 1–5 with tracheal structures and single elongate spiracle; first female endopodite absent, first exopodite vestigial. Endopodite 1 in males slightly curved outward distally, 3.7 times as long as wide, distal part without spines; exopodite 1 in males asymmetric, 1.5 times as long as wide, with narrower inner margin, tiny setae distributed along entire margin, 1 larger apical seta on inner narrow margin, 3 subapical setae, 1 seta on outer margin and 1 submarginal seta one-third before apex. Exopodite 2 in males very wide basally and narrow distally, 1.8 times as long as wide, with 5 submarginal setae; inner margin with tiny setae and notch in middle region; endopodite 2 in males slightly surpassing tip of exopodite 2 and with 2 setae on outer margin near median region (one of them long and slender and the other short and spatulate). Exopodites 3–4 with 3 submarginal setae near outer-distal margin. Exopodite 5 widely convex, with 4 submarginal setae near outer-distal margin.
Tegument ( Figs. 4–6 View Figure 4 View Figure 5 View Figure 6 ). Dorsal tegument of cephalothorax, pereon and pleon, as well as that of second antenna, pereiopods and maxilliped basipodite, bearing scaly microsculpture, with tricorn-like setae on some areas.
Variation. Adult size varied from 5.6–8.5 mm in total length, but males (5.6–7.2, n = 3) tend to be smaller than females (5.7–8.5, n = 6) in the sample; juveniles measured 4.8 and 5.0 mm. Two adult females had a small paramedian spine on one side of pleonite 5. One of the juveniles had a very small supernumerary spiniform tubercle between one of the pairs of paramedian tubercles of pereonite 1. The middle article of maxilliped palpus sometimes with two setae in distal position on the medial margin.
Natural history. Specimens of A. richardsonae sp. nov. were collected under rocks and in humid leaf litter in closed broadleaf forest, specifically in upper montane rainforest (F.Cala, in litt., 4.IX.2018) ( Fig. 9 View Figure 9 ).
Distribution. Know only from the type locality, around the ranger station, Blue Mountains, Saint Thomas Parish, Jamaica ( Fig. 1 View Figure 1 ). Nonetheless, on November 19 th, 2013, a specimen which at first sight matched the description of A. richardsonae sp. nov., was found in a leaf litter sample, photographed alive, and posted on iNaturalist by Sarah C. Crews (https://www.inaturalist.org/observations/484229; S.C. Crews, in litt. 24.IX.2021), from near Hardwar Gap( 18°08’05.6”N, 76°43’48.0”W; approximately 980 m a.s.l.), Blue and John Crow Mountains National Park, Portland Parish ( Figs. 1 View Figure 1 , 10 View Figure 10 ).
Etymology. The selected epithet is a Latinized patronym honoring Harriet Richardson Searle (1874–1958), Washington D.C., U.S.A., known as the first lady of isopods and one of the first women carcinologists, who made important contributions to isopod taxonomy and made the first description and illustrations of A. richardsonae sp. nov., although misidentified as A. spiniger .
Color (in alcohol) ( Fig. 3 View Figure 3 ). Grayish-brown, with muscle spots whitish-beige on dorsal surface of cephalothorax and on dorsolateral region of pereon tergites, around the base of largest dorsolateral spines; dorsal surface of pereon epimera and pleon tergites uniformly grayish-brown; dorsal surface of pereon epimera 1 sometimes spotted on dark gray. Second antenna uniformly light brown. Pereiopods grayish-brown irregularly spotted on whitish-beige. Apparently, the coloration in life is similar to that in alcohol ( Fig. 10 View Figure 10 ).
Remarks. The isopods of the genus Acanthoniscus are very fragile, therefore, most of the available specimens are fragmented, some of them severely damaged. Our description of A. richardsonae sp. nov. largely coincides with that of Richardson (1909), but since the precise locality of her specimen is unknown, we ignore whether its origin is the same as that of the type series of A. richardsonae sp. nov. (eastern Jamaica). Richardson (1909: 432) mentioned that her specimen had the cephalothorax “with the front emarginate and the lateral angles acutely produced” ( Fig. 4O View Figure 4 ). However, since all other overall characters she described coincide with the description of A. richardsonae sp. nov., we suspect that a different angle while making the drawings could be the cause for such differences.
Conservation status. Apparently, both A.spiniger and A. richardsonae sp. nov. have restricted distributions (known only from a single or two localities), with severely fragmented and reduced habitats. However, since only three collection events (two in the mid-19 th century) and an additional observation exist, it would be premature to make an evaluation of their conservation statuses based on the available information. Therefore, we tentatively consider them as Data Deficient ( IUCN Standards and Petitions Subcommittee, 2019). The type locality of A. richardsonae sp. nov. falls within the Blue and John Crow Mountains National Park (see Protected Areas System Master Plan: Jamaica 2013–2017; Jamaica’s National Ecological Gap Assessment Report, 2009), but that of A. spiniger is not included in any protected area.Additional surveys are required to collect data on their distributions, ecology, demographic parameters and threats, in order to make accurate assessments of their conservation statuses.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Oniscidea |
|
Family |
|
|
Genus |
Acanthoniscus richardsonae
| Rodríguez-Cabrera, Tomás M. & de Armas, Luis F. 2023 |
Acanthoniscus spiniger
| , White, List 1847 |