Nanomysis siamensis W.M. Tattersall, 1921
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5125.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:00C1EB04-34B0-4009-9BD3-60E0429A050B |
|
DOI |
https://doi.org/10.5281/zenodo.6425121 |
|
persistent identifier |
https://treatment.plazi.org/id/03E787C1-FFA1-FF95-19AD-27D5FF0D5098 |
|
treatment provided by |
Plazi |
|
scientific name |
Nanomysis siamensis W.M. Tattersall, 1921 |
| status |
|
Nanomysis siamensis W.M. Tattersall, 1921 View in CoL
Figs 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5
Nanomysis siamensis W.M. Tattersall, 1921: 409–410 View in CoL , pl. 15, figs. 7–10; –– O.S. Tattersall, 1960: 179 (in part).
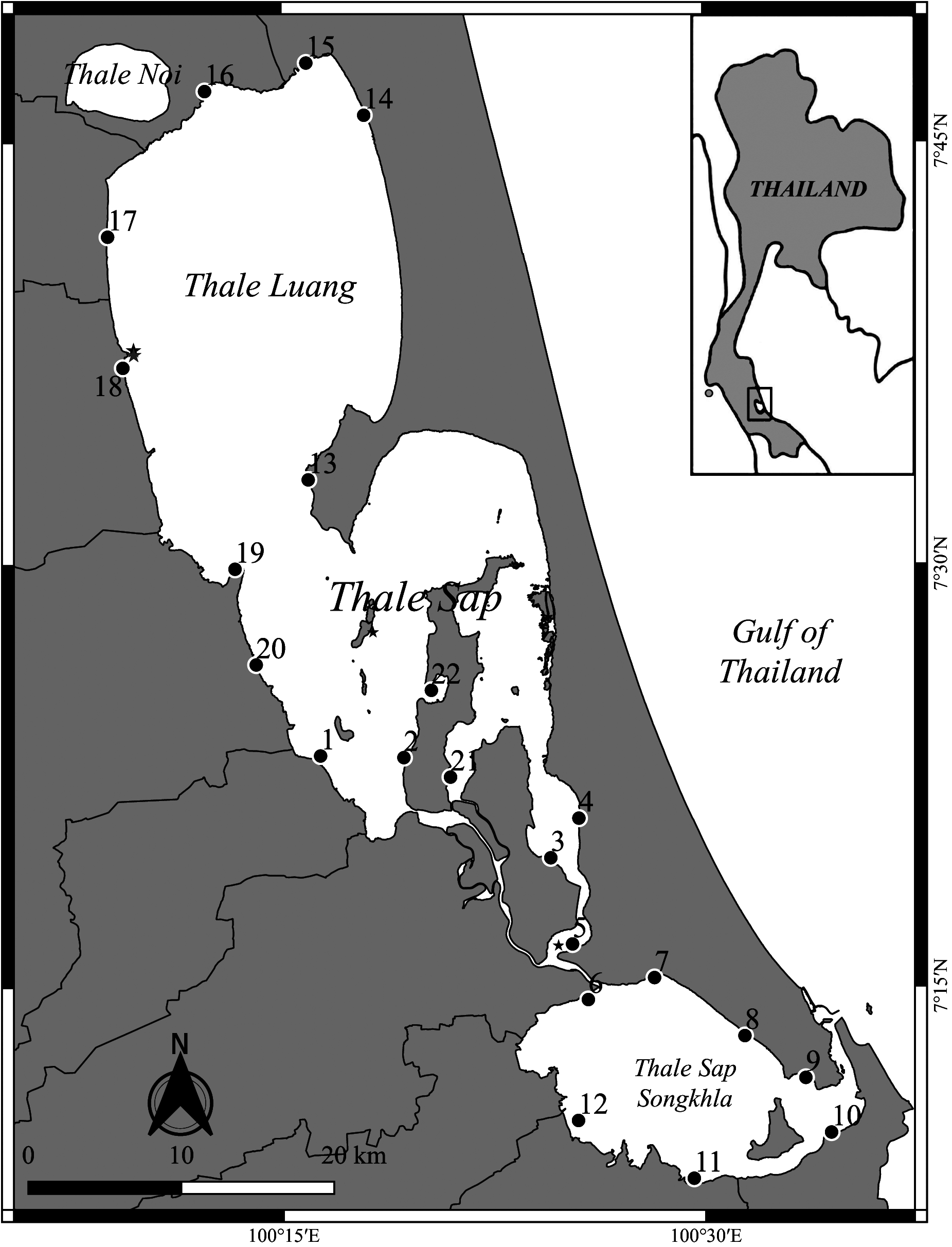
Type locality. A little south of the mouth of the Phatthalung river, Thale Luang (formerly known as Talé Sap), Siam (W.M. Tattersall 1921) .
Material examined. In the following study we chose to base our description on mature adults only. Detailed station data is presented in Table 1 View TABLE 1 .
Station 1. Thale Sap (7°23’12.62”N, 100°16’21.06”E), 12 adult males (BL 3.4–4.8 mm), 10 adult females with empty marsupium (BL 3.3–5.4 mm), two ovigerous females with eyeless larvae (BL. 4.1 and 4.2 mm) and two ovigerous females with eyed larvae (BL. 4.2 and 4.3 mm).
Station 2. Thale Sap (7°23’8.71”N, 100°19’18.72”E), five adult males (BL 3.5–4.3 mm), four adult females with empty marsupium (BL 3.6–4.3 mm) and one ovigerous female with egg (BL. 4.0 mm).
Station 3. Thale Sap (7°19’34.50”N, 100°24’31.45”E), 10 adult males (BL 3.1–4.0 mm), four adult females with empty marsupium (BL 3.7–4.2 mm), four ovigerous females with egg (BL 3.3–4.1 mm) and two ovigerous females with eyeless larvae (BL 3.1 & 4.2 mm).
Station 4. Thale Sap (7°20’58.68”N, 100°25’31.56”E), five adult males (BL 3.5–4.1 mm), one ovigerous female with egg (BL 4.0 mm), one ovigerous female with eyeless larvae (BL 4.0 mm) and three ovigerous females with eyed larvae (BL 3.8–4.7 mm).
Station 5. Thale Sap, (7°16’30.89”N, 100°25’17.21”E), 11 adult males (BL 3.3–4.6 mm), seven adult females with empty marsupium (BL 3.8–4.6 mm), one ovigerous female with egg (BL 4.3 mm), two ovigerous females with eyeless larvae (BL 4.9 & 5.0 mm) and two ovigerous females with eyed larvae (BL 4.2 & 4.5 mm).
Station 6. Thale Sap Songkhla (7°14’32.41”N, 100°25’50.57”E), five adult males (BL 3.3–3.7 mm), two ovigerous females with egg (BL 3.4 & 3.8 mm), one ovigerous female with eyeless larvae (BL 3.6 mm) and two ovigerous females with eyed larvae (BL 3.8 & 4.2 mm) GoogleMaps .
Station 7. Thale Sap Songkhla (7°15’18.77”N, 100°28’11.86”E), five adult males (BL 3.7–4.4 mm), three adult females with empty marsupium (BL 4.3–4.9 mm) and two ovigerous females with eyeless larvae (BL 4.4 & 4.8 mm) GoogleMaps .
Station 8. Thale Sap Songkhla (7°13’14.67”N, 100°31’24.12”E), five adult males (BL 3.7–4.1 mm) and five adult females with empty marsupium (BL 4.4–4.6 mm) GoogleMaps .
Station 9. Thale Sap Songkhla (7°11’45.16”N, 100°33’33.76”E), five adult males (BL 3.4–4.0 mm), one adult female with empty marsupium (BL 4.9 mm), two ovigerous females with egg (BL 4.2 & 4.3 mm) and two ovigerous females with eyed larvae (BL 4.0 & 4.3 mm) GoogleMaps .
Station 10. Thale Sap Songkhla (7°09’48.71”N, 100°34’28.17”E), five adult males (BL 3.4–4.2 mm) and five adult females with empty marsupium (BL 3.8–4.7 mm) GoogleMaps .
Station 11. Thale Sap Songkhla (7°8’11.31”N, 100°29’35.21”E), five adult males (BL 3.3–3.4 mm) and five adult females with empty marsupium (BL 3.5–4.5 mm) GoogleMaps .
Station 12. Thale Sap Songkhla (7°10’15.03”N, 100°25’28.78”E), five adult males (BL 3.4–4.2 mm), three ovigerous females with egg (BL 3.6–3.8 mm) and two ovigerous females with eyeless larvae (BL 4.0 & 4.2 mm) GoogleMaps .
Station 13. Thale Luang (7°33’1.70”N, 100°15’56.30”E), two adult males (BL 3.4 & 4.2 mm), four adult females with empty marsupium (BL 4.1–4.6 mm) and one ovigerous female with egg (BL 4.6 mm).
Station 14. Thale Luang (7°45’57.70”N, 100°17’57.00”E), 10 adult males (BL 2.9–4.2 mm), six adult females with empty marsupium (BL 3.1–4.0 mm), three ovigerous females with egg (BL 3.0– 3.4 mm) and one ovigerous female with eyeless larvae (BL 3.5 mm).
Station 15. Thale Luang (7°47’49.20”N, 100°15’53.80”E), six adult males (BL 3.3–4.0 mm), one adult female with empty marsupium (BL 3.4 mm), one ovigerous female with egg (BL 3.3 mm), two ovigerous females with eyeless larvae (BL 3.6 & 3.7 mm) and one ovigerous female with eyed larvae (BL 3.4 mm).
Station 16. Thale Luang (7°46’49.10”N, 100°12’17.40”E), five adult males (BL 3.0– 4.2 mm), two adult females with empty marsupium (BL 3.4 & 3.5 mm), two ovigerous females with egg (BL 3.3 & 3.3 mm) and one ovigerous female with eyeless larvae (BL 3.1 mm).
Station 17. Thale Luang (7°41’39.50”N, 100°08’49.60”E), five adult males (BL 3.1–4.1 mm), one adult female with empty marsupium (BL 3.7 mm), two ovigerous females with egg (BL 3.1 & 3.3 mm) and two ovigerous females with eyeless larvae (BL 3.2 & 3.3 mm).
Station 18. Thale Luang (7°37’0.30”N, 100°09’21.50”E), six adult males (BL 3.3–3.7 mm), one adult female with empty marsupium (BL 3.5 mm), one ovigerous female with egg (BL 3.2 mm), two ovigerous females with eyeless larvae (BL 3.5 & 3.5 mm) and two ovigerous females with eyed larvae (BL 3.4& 3.5 mm).
Station 19. Thale Luang (7°29’51.80”N, 100°13’19.60”E), one adult male (BL 4.3 mm), one adult female with empty marsupium (BL 4.5 mm), one ovigerous female with egg (BL 4.2 mm) and two ovigerous females with eyeless larvae (BL 4.2 & 4.5 mm).
Station 20. Thale Sap (7°26’26.80”N, 100°14’4.30”E), seven adult males (BL 3.2–4.3 mm), five adult females with empty marsupium (BL 3.3–4.5 mm), one ovigerous female with egg (BL 4.5 mm) and one ovigerous female with eyeless larvae (BL 4.3 mm).
Station 21. Thale Sap (7°22’26.90”N, 100°20’58.10”E), six adult males (BL 3.4–3.8 mm), two ovigerous females with egg (BL 3.6 & 3.7 mm), two ovigerous females with eyeless larvae (BL 4.2 & 4.2 mm) and one ovigerous female with eyed larvae (BL 4.1 mm).
Station 22. Thale Sap (7°25’31.80”N, 100°20’17.80”E), five adult males (BL 3.6–4.5 mm), one adult female with empty marsupium (BL 3.3 mm), one ovigerous female with egg (BL 3.6 mm) and four ovigerous females with eyeless larvae (BL 4.0 – 4.6 mm).
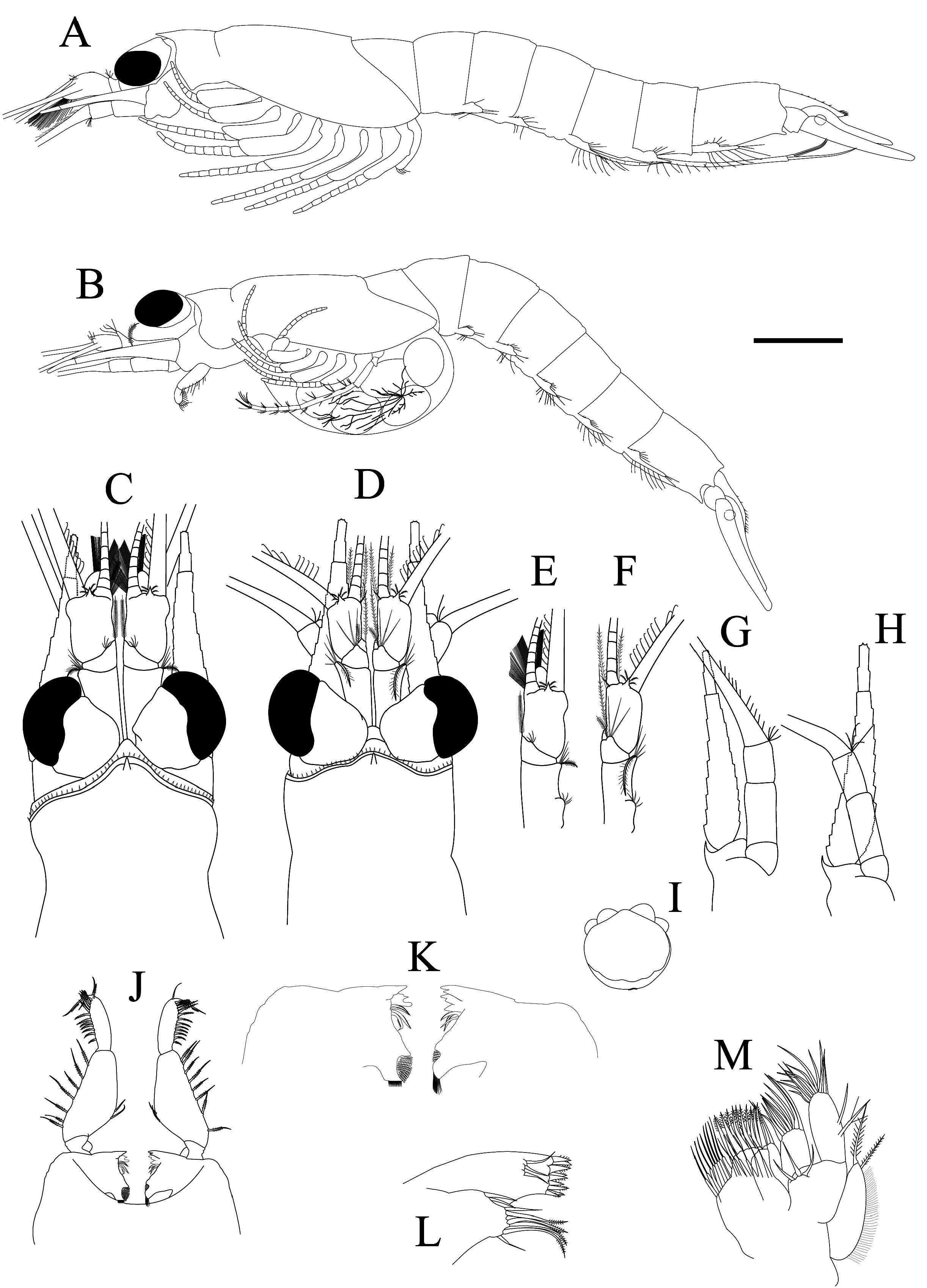
Description. Head and cephalic appendages: carapace with anterior margin obtusely produced into wide, subtriangular or rounded rostrum, frontal margin fringed with several short spines and bearing single stout triangular median process ( Fig. 2C, D View FIGURE 2 ); cervical groove distinct at anterior 1/4 of carapace, posterior margin excavated, leaving last thoracic somite uncovered in dorsal view, but sufficiently covered laterally: antero-ventral corner rounded ( Fig. 2A, B View FIGURE 2 ).
Eyes: slightly depressed dorsoventrally and slightly longer than wide, cornea occupying two-fifths of whole eye in dorsal view ( Fig. 2C, D View FIGURE 2 ).
Antennule: antennular peduncle of male; first article 1.7 times as long as broad, four hooked setae present at about 1/3 part of lateral margin, distolateral corner armed with four short simple setae and one long curved plumose seta; second article shortest, with dorsal projection bearing four short simple setae and distal part with one plumose seta; third article 1.5 times as long as broad, with short triangular median process and four short plumose setae, inner lateral flagellum swollen at basal part, forming male lobe and its mesial margin densely hirsute with long setae ( Fig. 2C, E View FIGURE 2 ); antennular peduncle of female more slender than male, first article 2.3 times as long as broad, four hooked setae at posterior 1/3 part of lateral margin, distolateral corner armed with four short simple setae and one long curve plumose seta; second article shortest, with dorsal projection bearing four simple setae, which are longer than male, and distal part with one plumose seta; third article 2.0 times as long as broad, with short triangular median process and four short plumose setae, distomesial corner with two plumose setae and one short simple seta ( Fig. 2D, F View FIGURE 2 ).
Antenna: antennal scale slender, lanceolate, slightly longer than antennular peduncle and about 6.2 to 6.8 times as long as broad, setose all around, with apical suture; antennal peduncle extending middle part of antennal scale; first article shortest; second article about 1.5 times as long as broad in male and slightly longer than in female; third article 2/ 3 in length of second article, with four setae; antennal sympod with spine-like process at distal corner ( Fig. 2G, H View FIGURE 2 ).
Labrum: subglobular in shape, asymmetric, anterior part with short projection, distolateral corner with 2 pairs of projections, posterior part with shallow depression bearing very smooth setae ( Fig. 2I View FIGURE 2 ).
Mandible: mandibular palp with three articles; first article shortest; second article longest and widened at middle, with barbed setae on both margins, two at middle part of inner margin and seven or eight setae on outer external margin; third article about 1/3 length of the second article, armed with 14 short barbed setae, one long barbed seta and one short seta ( Fig. 2J View FIGURE 2 ); incisor process well-developed and comprised of a series of teeth forming serrated sharp ridge; lacinia mobilis showing different shapes in right and left mandibles, and spine row and molar process clearly visible ( Fig. 2K View FIGURE 2 ).
Maxillule: well-developed, basal lobe with nine stout spines on apical margin and three setae on its surface; precoxal lobe with five long barbed setae and four simple setae ( Fig. 2L View FIGURE 2 ).
Maxilla: exopod slender, reaching distal article of endopod, outer margin with numerous smooth setae, one short plumose seta and apical margin with one long plumose seta; distal segment of endopod longer than proximal one; basal and coxal endites well-developed, with dense setae ( Fig. 2M View FIGURE 2 ).
Thoracopods: flagelliform part of first ( Fig. 3A View FIGURE 3 ) and eighth thoracopodal exopods ( Fig. 4D View FIGURE 4 ) composed of eight articles, while second ( Fig. 3C View FIGURE 3 ) to seventh thoracopodal exopods ( Fig. 4C View FIGURE 4 ) with nine articles. First thoracopodal endopod (maxilliped 1) short and basis well developed, larger than endite; medial margins of carpus, propodus and dactylus heavily setose ( Fig. 3A, B View FIGURE 3 ). Second thoracopodal endopod (maxilliped 2) stout; basis with two setae; preischium shortest with two setae; ischium longer than preischium with seven setae; merus longest with four setae; carpopropodus 0.8 times as long as merus, with several barbed setae; dactylus 0.4 times as long as carpopropodus with several barbed setae ( Fig. 3C, D View FIGURE 3 ). Third to seventh thoracopodal endopods (pereopods) similar in form ( Figs. 3E, F View FIGURE 3 , 4A–C View FIGURE 4 ) and more slender than second ( Fig. 3C View FIGURE 3 ); basis with one or two plumose setae; preischium shortest with one or two setae; ischium of third to fifth thoracopodal endopods subequal in length to merus and the sixth to seventh thoracopodal endopods slightly longer than merus; ischium and merus armed with several setae; carpopropodus constituting three sub-segments with several setae; dactylus with long stout nail with several setae. Eighth thoracopodal endopod longest; basis with one simple seta and one plumose seta; preischium shortest; ischium about half of merus with one simple seta; merus armed with several setae and one antler-shaped spine; carpopropodus constituting four sub-segments with three antler-shaped spines in male ( Fig. 4D View FIGURE 4 ) and four spines in female ( Fig. 2B View FIGURE 2 ); dactylus with long stout nail with several setae ( Fig. 4D View FIGURE 4 ). Penis short ( Fig. 4E View FIGURE 4 ), about 2.5 times as long as wide and armed with four curved setae on the apical margin.
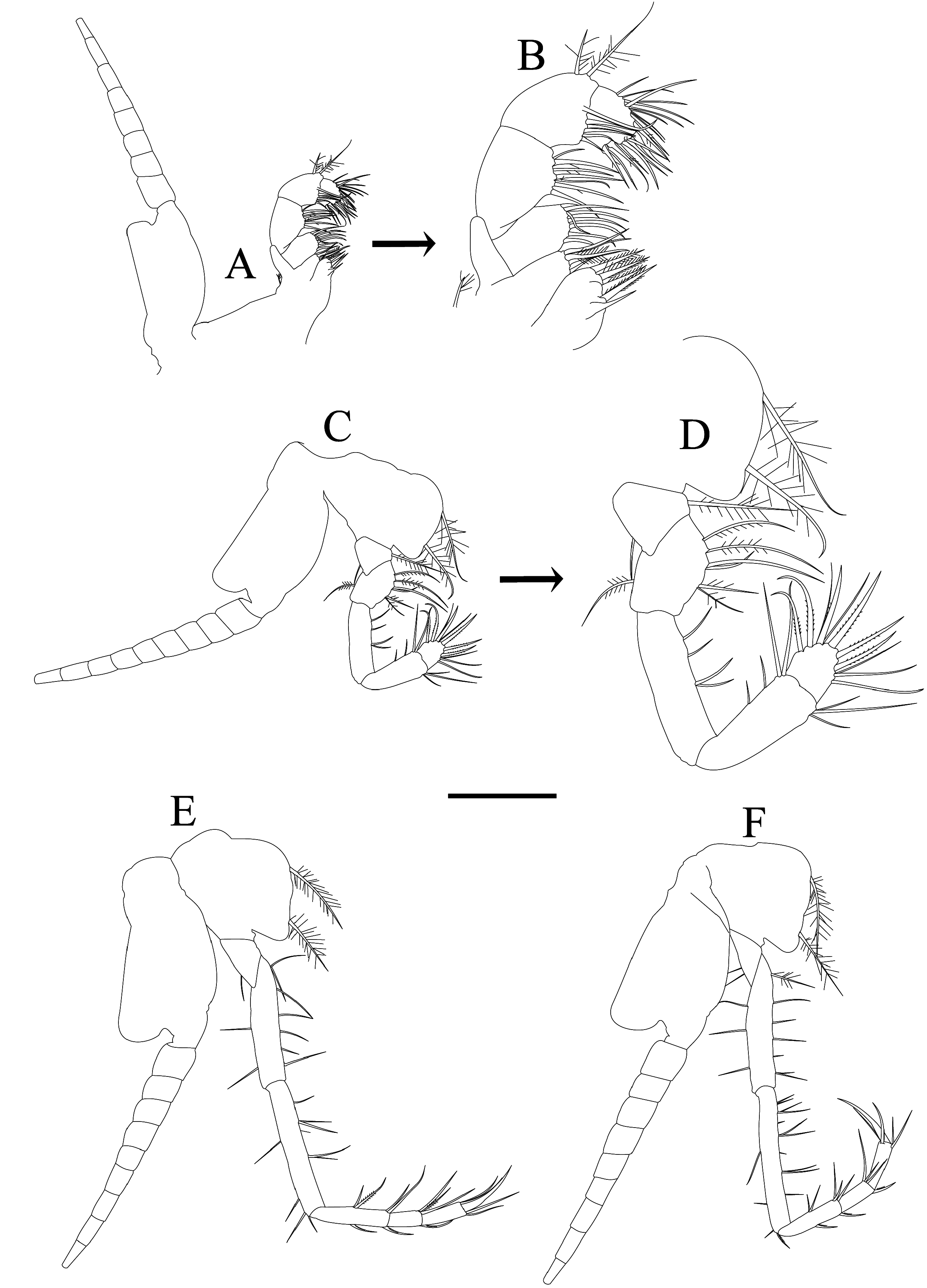
Pleon and pleopods: abdominal somites smooth, without hairs, spines or folds, ventral sternites without process; first and second somites subequal in length, third and fifth somites subequal in length and slightly shorter than preceding two, fourth somite shortest, sixth somite 1.3 times as long as preceding ( Fig. 2A, B View FIGURE 2 ); first, second and fifth male pleopods rudimentary, unsegmented, gradually increasing in length posteriorly with several setae ( Fig. 5A, B, E View FIGURE 5 ); third male pleopod biramous ( Fig. 5C View FIGURE 5 ), length subequal to sixth abdominal somite; endopod shorter than exopod, unsegmented, bearing several setae on outer and inner margins; exopod slender, straight or sometimes curving comprising three articles; first article longest about twice as long as endopod and possessing three setae, second article with one seta and terminal article shortest, with one stout seta; fourth pleopod longest, biramous ( Fig. 5D View FIGURE 5 ); endopod short, unsegmented, bearing several setae at the inner and outer margins; exopod slender, straight or sometimes curving near base, with four articles, extending posteriorly to distal end of telson, first article about twice as long as endopod with five setae, second article slightly longer than third and without setae, third article with one stout seta, where the seta extend about 1.5 times of the third article with several smooth setae from posterior 1/3 to terminal part of seta, fourth article shortest, with two setae bearing smooth setae at terminal; first to fifth female pleopods rudimentary, unsegmented, gradually increasing in length posteriorly with several setae ( Fig. 5F–J View FIGURE 5 ).
Uropod and telson: uropodal endopod about 0.7 times as long as exopod; slightly more than 1.5 times as long as telson, without spine on inner ventral side of statocyst region ( Fig. 5K View FIGURE 5 ); telson trapezoid ( Fig. 5L–O View FIGURE 5 ), 0.8 times as long as sixth abdominal somite, 2.2 times as long as broad at basal part; lateral margin armed with 5–9 sharp spines in male and 8–10 in female; posterior margin convex, armed with two large spines at lateral corners and 11–15 apical spines in males and 15–17 apical spines in females.
Body length. Male, 2.9–4.8 mm; female, 3.0– 5.4 mm.
Distribution. Nanomysis siamensis is considered a resident spesies in the Songkhla Lagoon System and was first recorded from Thale Luang and waters of the Thale Sap (W.M. Tattersall 1921). Recently, the distribution of N. siamensis was expanded southward by the discovery of populations in waters of Thale Sap Songkhla ( Lheknim & Yolanda 2020; Yolanda & Lheknim 2021).
Remarks. Due to having access to only a few specimens, the first description of N. siamensis by W.M. Tattersall (1921) was limited in presenting morphological variation. The type materials came from four stations, two sites close to the Phatthalung river, one at Koh Si Hah and one near Songkhla (formerly known as Singgora) (see Fig. 1 View FIGURE 1 ). These sites are close to the station 18 in our study for the Phatthalung river and station 5 near Singgora. To date, these areas are restricted and declared as protected area and part of concession for swallow bird’s nest.
In W.M. Tattersall’s (1921) original description, he found (1) the first and fourth abdominal somites of equal size and the shortest, (2) third to eighth thoracopodal endopods comprised of four carpopropodus, (3) the length of antennal scale about 7 times as long as wide, (4) the first article of male third pleopod without setae, (5) three setae at the first segment of the male fourth pleopod, and (6) telson armed with about 10 spines along the lateral margins and an additional large spine at the outside corners of the apex which armed with a comb of 12 spines. Comparing Tattersall’s description to our study, based on 272 specimens we found some disdepancies reflecting some interspecific variation (1) the first and second abdominal somites subequal and fourth somite shortest ( Fig. 2A, B View FIGURE 2 ), (2) third to seventh thoracopodal endopod comprised of three-segmented carpopropodus ( Figs. 3E, F View FIGURE 3 , 4A– C View FIGURE 4 ), and only the eighth with four-segmented carpopropodus with three or four antler-shaped spines ( Fig. 4D View FIGURE 4 ), (3) antennal scale is about six to seven times as long as wide ( Fig. 2G, H View FIGURE 2 ), (4) three setae in the first article of the male third exopodal pleopod ( Fig. 5C View FIGURE 5 ), (5) five to seven setae in the first article of the male fourth exopodal pleopod ( Fig. 5D View FIGURE 5 ), and (6) telson armed with 5–13 lateral spines and also with 8–21 spines in the apical margin ( Fig. 5K–O View FIGURE 5 ).
Murano (1997) compared N. philippinensis with N. siamensis and N. insularis on the five characteristics; (a) the first segment of the exopod of the male third pleopod, (b) the first segment of the exopod of the male fourth pleopod, (c) the posterior margin of the telson and number of spines, (d) the number of spines on the lateral margin of the telson, and (e) the body length. He subsequently modified the generic diagnosis by the addition of the following three characters states: the anterior margin of the carapace fringed with spinules; the thoracic endopods with 3- or 4– jointed carpopropodus; and the posterior margin of telson convex or straight or concave, armed with a comb of spines. However, as already stated it is only the eighth thoracopod that bears a 4-segmented carpopropodus.
N. siamensis may be distinguished from N. philippinensis by the following characters; (a) anterior median part of the carapace bearing short stout triangular process, while no process in N. philippinensis , (b) precoxal lobe of the maxillule bearing five long barbed and four simple setae, while three long stout and six simple setae in N. philippinensis , (c) the adult males and females of N. siamensis were larger than N. philippinensis , (d) telson armed with 5–13 lateral spines and convex posterior margin with 8–21 spines, while N. philippinensis 5–9 lateral spines concaved posterior margin with 5–10 spines, (e) first and eighth thoracopodal exopods with eight articles, while those of second to seventh with nine articles, while first to seventh thoracopodal exopods with nine and that of eighth with eight articles in N. philippinensis .
Nanomysis siamensis also differs from N. insularis where (a) the eye is slightly depressed, sub-quadrangular and cornea about 2/5 of whole eye from dorsal aspect, while it is globular and cornea about 1/2 of the whole eye in N. insularis , (b) 1/3 part lateral margin of the antennular peduncle armed with four hooked setae in male and female, while only two hooked setae present in female of N. insularis , (c) outer margin of the second segment of the mandibular palp bearing seven or eight setae, while 13 setae in N. insularis , (d) telson armed with 5–13 lateral spines and convex posterior margin with 8–21 spines, while N. philippinensis 11–12 lateral spines concaved posterior margin with 15 spines. Detailed morphological differences among the three Nanomysis species is presented in Table 2 View TABLE 2 .
Gangemysis assimilis (W.M. Tattersall, 1908) , a similar-sized mysid occurring in the Asian waters to Malay Peninsula (W.M. Tattersall 1908, 1914, 1915, 1922; Hanamura et al. 2008b), superficially resembles N. siamensis . However, these two species can be distinguished as follows; (a) biramous third male pleopod well-developed and subequal in length to the sixth abdominal somite in N. siamensis , while in G. assimilis , it is rudimentary about half the length of the fourth abdominal somite, (b) the fourth male pleopod extending to the uropod in N. siamensis , while in G. assimilis , it extends to the middle of the sixth abdominal somite, (c) telson armed with 5–13 lateral spines and convex posterior margin with 8–21 spines and two large spines in N. siamensis , while 7–10 lateral spines and 9–10 short spines and four large posterior spines in G. assimilis .
Distribution of N. siamensis in the Songkhla Lagoon System seems to be primarily influenced by salinity. The four main water bodies in the Songkhla Lagoon System have different salinity regimes: Thale Noi always freshwater, Thale Luang and Thale Sap from freshwater to brackish water and Thale Sap Songkhla from freshwater to seawater. When first discovered, W.M. Tattersall (1921) collected his specimen during the flooding season of the Lagoon system in January 1916. He then reported that N. siamensis was more abundant in Thale Luang (the inner lake) than in Thale Sap (the middle lake). Tattersall registered that in the inner lake the water was quite fresh, whereas outer lake was slightly brackish with a salinity at approximately 7.0 psu.
Comparing Tattersall’s observations to the present distribution, Yolanda & Lheknim (2021) reported that N. siamensis is now more abundant in Thale Sap than in Thale Sap Songkhla. These observations are further confirmed in the present study where this species has a higher abundance in Thale Sap compared to Thale Luang and also to Thale Sap Songkhla ( Fig. 6 View FIGURE 6 ). However, although sampling in Thale Noi has not been conducted, yet. Based on laboratory observations, we have seen that N. siamensis cannot survive in freshwater (salinity 0) for more than 24 hours (Yolanda 2021). In effect, based on the distribution presented in our study it seems that N. siamensis prefers slightly brackish water, more so than seawater, and has a low tolerance to decreased salinity values, and we therefore do not expect to find this species the freshwater lagoon Thale Noi.
With regard to salinity, it is interesting to note that during Tattersalls’ sampling in 1916 hydrographical properties in the lakes of the Songkhla Lagoon was most likely quite different from what we have in 2022. During our sampling we met and talked to local people that through generations have lived on and around the Songkhla Lagoon. Sharing their knowledge on the history of the lagoon it was interesting to learn that in 1916 there were a multitude of manmade water channels that connected the Songkhla Lagoon with the Gulf of Thailand, especially along the coast, between the Gulf and Thale Luang, Thale Sap, and Thale Sap Songkhla. In effect it is likely that, 100 years ago, the inner and outer lakes where more similar in salinity, due to the direct contact of the entire system to saline gulf water through constructed water channels. To what degree the water channels have influenced the distribution of mysids in the Songkhla Lagoon is difficult to say, but we do know that from 1950 to present the channels have gradually been removed due to the establishment of settlements and housing. And in removing a direct contact between saline and freshwater there in no doubt that the hydrography of these three lakes has changed from 1916 to 2021, and we do suspect that the human impact on the lagoon has influenced the distribution of E. siamensis since its first finding in 1916 .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nanomysis siamensis W.M. Tattersall, 1921
| Yolanda, Rofiza, Sawamoto, Shozo & Lheknim, Vachira 2022 |
Nanomysis siamensis W.M. Tattersall, 1921: 409–410
| Tattersall, O. S. 1960: 179 |
| Tattersall, W. M. 1921: 410 |