Pseudogaurax idiogenes Wheeler
|
publication ID |
https://doi.org/ 10.5281/zenodo.185076 |
|
DOI |
https://doi.org/10.5281/zenodo.6225948 |
|
persistent identifier |
https://treatment.plazi.org/id/03E73656-990F-ED12-FF53-B1C1FABDFA2D |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudogaurax idiogenes Wheeler |
| status |
sp. nov. |
Pseudogaurax idiogenes Wheeler View in CoL , sp. n.
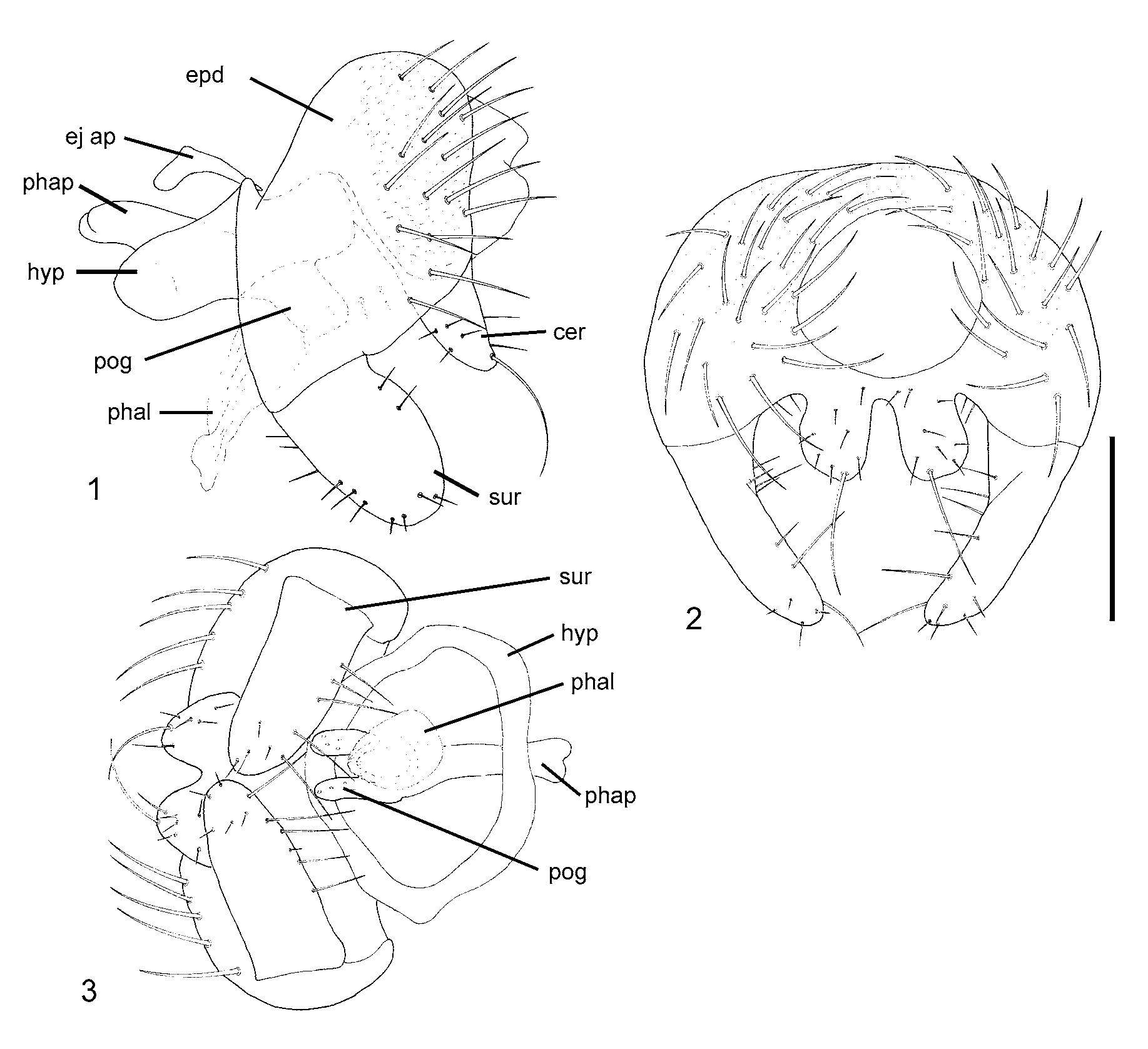
( Figs. 1–3 View FIGURES 1 – 3 )
Description: Total length 3.0– 3.5 mm. Frons yellow, subequal in width to eye in dorsal view; frontal triangle half as long as frons, shining yellow, ocellar tubercle shining black; 8–10 long, pale fronto-orbital setae; several pale interfrontal setae almost as long as fronto-orbital setae; ocellar bristles short, reclinate; vertical and postocellar bristles long, pale; eye large, densely hairy; gena yellow, genal height 0.1 times eye height; vibrissa and subvibrissal setae long, pale; postgena yellow, narrow; occiput yellow; face flat, pale yellow; pedicel yellow, first flagellomere yellow, reniform, higher than long; arista long pubescent, dark brown; proboscis small, pale; palpus yellow, with long, pale distal and ventral setulae.
Thorax with pronotum distinct in dorsal view, pale yellow; scutum yellow with dark yellow to orange longitudinal median and intra-alar stripes, median stripe with narrow, darker orange stripe on either side of midline in some specimens, small, dark, medial spot anterior to scutellum; scutal setae pale, postpronotal and notopleural setae slightly darker; 1 anterior and 2 posterior notopleural setae; row of prescutellar setae slightly longer than other scutal setulae; scutellum yellow, rugose, with long pale setulae; apical scutellar bristles pale, divergent, strong, lateral scutellar setae weak, pale; thoracic pleurites yellow except for dark shining anteroventral spot on anepisternum. Legs pale yellow except for brown band on middle third of hind tibia; femoral organ absent; tibial organ large, oval, pale, interrupting brown tibial band on posterior surface of hind tibia. Wing typical of the genus; second costal sector 1.7–2.0 times as long as third, cell c broad; halter yellow.
Abdominal syntergite 1+2 yellow with brown posterolateral corners, tergites 3–5 brown, tergite 3 sometimes paler medially; tergites with long weak setae, especially laterally.
Male postabdomen ( Figs. 1–3 View FIGURES 1 – 3 ): epandrium pale yellow, broader than high in posterior view; surstylus straight, slightly clavate, setose and setulose; hypandrium short and broad in ventral view; postgonites wellsclerotized, broad and quadrate in lateral view; distiphallus long, pale, membranous; cerci long, quadrate, well-sclerotized, diverging apically and separated by deep U-shaped ventral cleft, each cercus with long ventral seta and shorter setae and setulae; subepandrial sclerite simple, pale.
Female postabdomen: tergite 6 well-sclerotized, with posteromedial dark spot; other tergites and sternites of segments 6–8 reduced; tergite 10 triangular, shining; cerci well-sclerotized, elongate, narrow in dorsal Etymology: The species name is from the Greek idiogenes (distinctive, peculiar), referring to the habits of the larvae, feeding on eggs of Megaloptera instead of spider egg masses like most species of this genus.
Comments: This species will not key out in Sabrosky’s (1966; 1990) keys to New World Pseudogaurax . It runs to couplet 13 in Sabrosky (1966) but does not key to either half of that couplet because of the color pattern of the legs. We have not included revised couplets to Sabrosky’s keys to species because the large number of undescribed Neotropical species of Pseudogaurax would make any effort premature pending a complete revision of the fauna. Specimens of P. idiogenes may be distinguished from other described New World species of Pseudogaurax by the combination of the scutal colour pattern, the yellow scutellum and the extensively yellow legs with a dark band on the hind tibia.
Natural history of Pseudogaurax idiogenes
Pseudogaurax idiogenes specimens were obtained from Megaloptera egg-masses collected in Parque Estadual Intervales, Iporanga, São Paulo, Brazil (24o17’36” S, 48o25’06”W, 600–700 m el.), on 26-I-2002. Egg-masses were located on a small wooden bridge with a foundation of sedimentary rocks vertical to the stream. Four egg-masses (~ 18 mm diameter each) were obtained from the bridge foundation. They were removed using a screwdriver and included a small piece of rock to which they were attached, thus preventing damage to the egg-masses. Two pieces of rock, each containing two egg-masses (~ 5 mm apart in each case) were collected. The white covering material of all egg-masses was homogenous and did not contain any sign of oviposition by Chloropidae . It is thus likely that female flies attacked the megalopteran egg-masses during or immediately after oviposition.
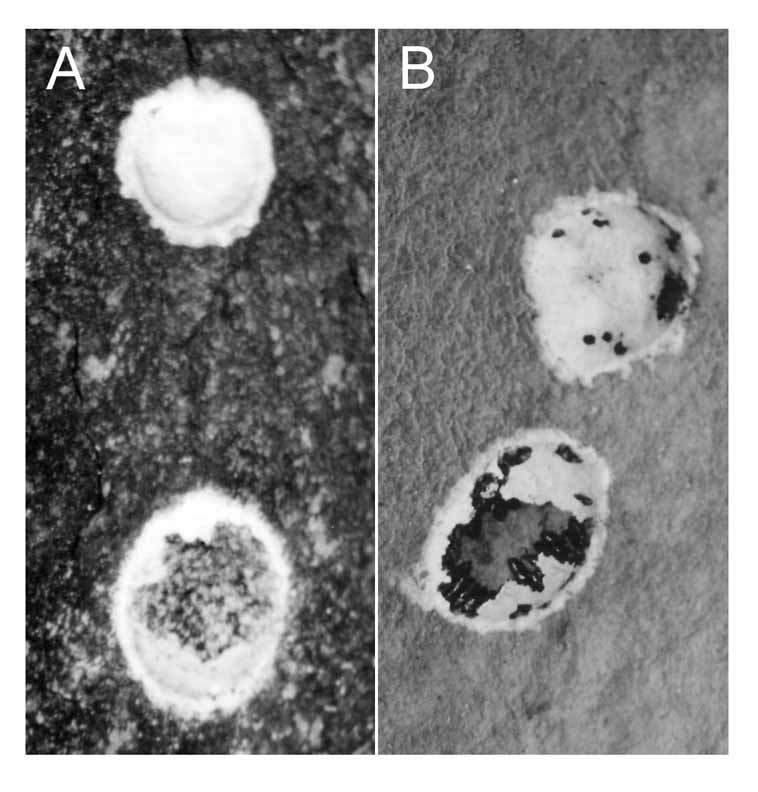
It is not certain to which species of Megaloptera the egg-masses belonged. No description of Megaloptera larvae is available for the species occurring in the region. However, adults of Chloronia corripiens (Walker) and Corydalus diasi Navás have been collected at the same site. A third species, Corydalus hecate MacLachlan , has been collected from two streams in the same watershed, ca. 10 km from the study site. Azevedo (2003) provides photographs of egg-masses of Corydalus sp. and Chloronia hieroglyphica (Rambur) from the central Amazon. Egg-masses of Corydalus are white, while those of Chloronia hieroglyphica are pale-brown. If coloration is diagnostic at the genus level, egg-masses from the study site probably belong to Corydalus diasi or Corydalus hecate ( Fig. 4 View FIGURE 4. A ).
Because the initial objective was to obtain megalopteran larvae, egg-masses were not separated in individual containers. On 31-I-2002 five flies emerged from one egg-mass. Flies were first seen at 06:50 am and it is likely that they emerged minutes before, as they were close to the egg-mass and the wings of one individual were not yet expanded. It was possible to identify the five small holes in the egg-mass from which they emerged. Two flies escaped during transfer between vials; the three remaining specimens were females. On 01-II-2002 2 males and 6 females emerged between 06:40 and 06:50 am from the same egg-mass. On 06-II- 2002 2 males and 3 females emerged between 07:50 and 08:25 am from the second egg-mass on the same piece of rock. On 07-II-2002 around 07:50 am, two further individuals (2 females) were obtained, likely from the second egg-mass. The last fly (1 female) emerged on 22-II-2002. It was not possible to determine its exact emergence time, although it was first sighted around 09:30 am. The puparia of reared flies remained inside the megalopteran egg-mass.
The two egg-masses on the second piece of rock produced no flies. Megalopteran larvae emerged from one of them in the night of 06-07-II-2002. The second egg-mass remained intact.
A second visit was made to the sample site on 09-III-2004 to assess the proportion of attacked egg-masses and the number of puparia per egg-mass. A total of 126 egg-masses was recorded. Nine were in poor condition, indicating that they were likely from the previous year. One intact egg-mass (no Megaloptera or Chloropidae emergence) was partially covered by the nest of a wasp, and one showed signs of fungal infection. Another egg-mass was damaged. From the remaining 114 egg-masses, two (1.8%) were pre-emergence, 24 (21.0%) showed signs of megalopteran emergence, and 88 (77.2%) contained fly puparia or small holes (sign of Chloropidae emergence) ( Fig. 4 View FIGURE 4. A B). Twenty egg-masses showing signs of Chloropidae attack were removed from the substrate, fixed in alcohol and returned to the laboratory to estimate the number of flies developing per egg-mass. The mean number of Chloropidae puparia per egg-mass was 26.65 (n = 20, min = 4, max = 77, sd = 20.62). Some of the egg-masses were attached to the lower surface of the substrate, and as the fly puparia are only loosely attached to the substrate, it is possible that some of them fell down before collection. Additionally, egg-masses revealed saprophagous invertebrates such as Acari and Psocoptera (Insecta), which may have destroyed part of the material. Thus the numbers provided above may be underestimates.
Five out of the 20 examined egg-masses contained dipteran larvae, likely from Chloropidae . In three eggmasses there were well-developed pupae and adults of small wasps. Each fly puparium was parasitized by a single wasp. One megalopteran egg-mass contained two adult wasps and 20 pupae of wasps. Additionally, in four egg-masses some fly puparia (1 of 18, 3 of 11, 2 of 50, and 60 of 77 puparia, respectively) were completely filled by small larvae, probably parasitoid Hymenoptera .
Although data are available only from a single locality in southeast Brazil, it is likely that this Chloropidae-Corydalidae host association occurs elsewhere. Some egg-masses of Corydalidae observed during a casual visit to a stream site in southern Brazil (Maquiné, Rio Grande do Sul, 29°31’33”S, 50°18’56”W, XII- 2004), ~ 700 km from the study site, contained small holes similar to those observed in this study.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |