Toxoniella, Warui, C. & Jocque, R., 2002
|
publication ID |
https://doi.org/ 10.5281/zenodo.819918 |
|
DOI |
https://doi.org/10.5281/zenodo.6279030 |
|
persistent identifier |
https://treatment.plazi.org/id/03E73070-FFAA-FFDE-FE2F-FE5DF1C41CFB |
|
treatment provided by |
Jeremy |
|
scientific name |
Toxoniella |
| status |
gen. nov. |
Toxoniella new genus
Type species. — Toxoniella taltensls new species.
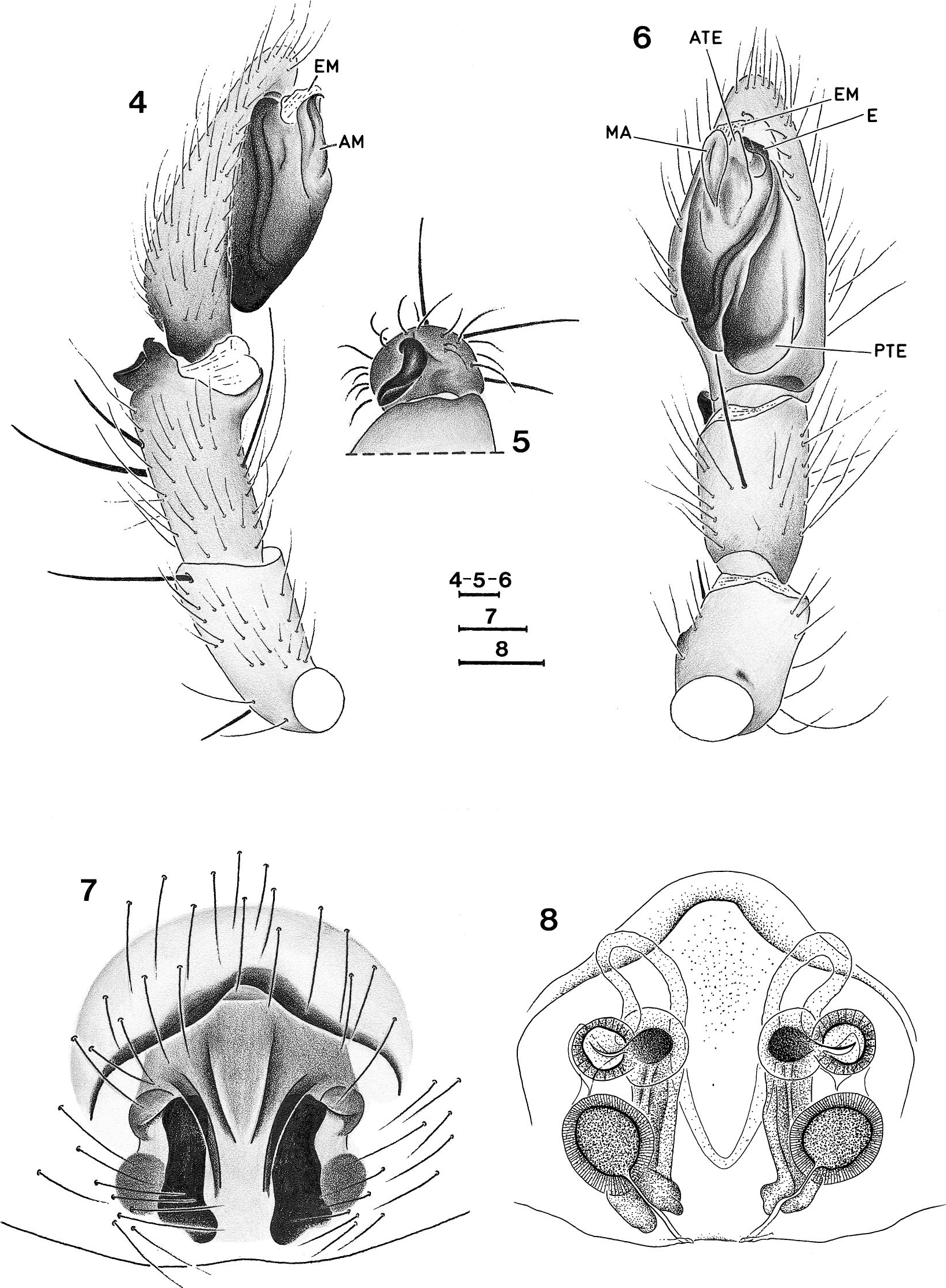
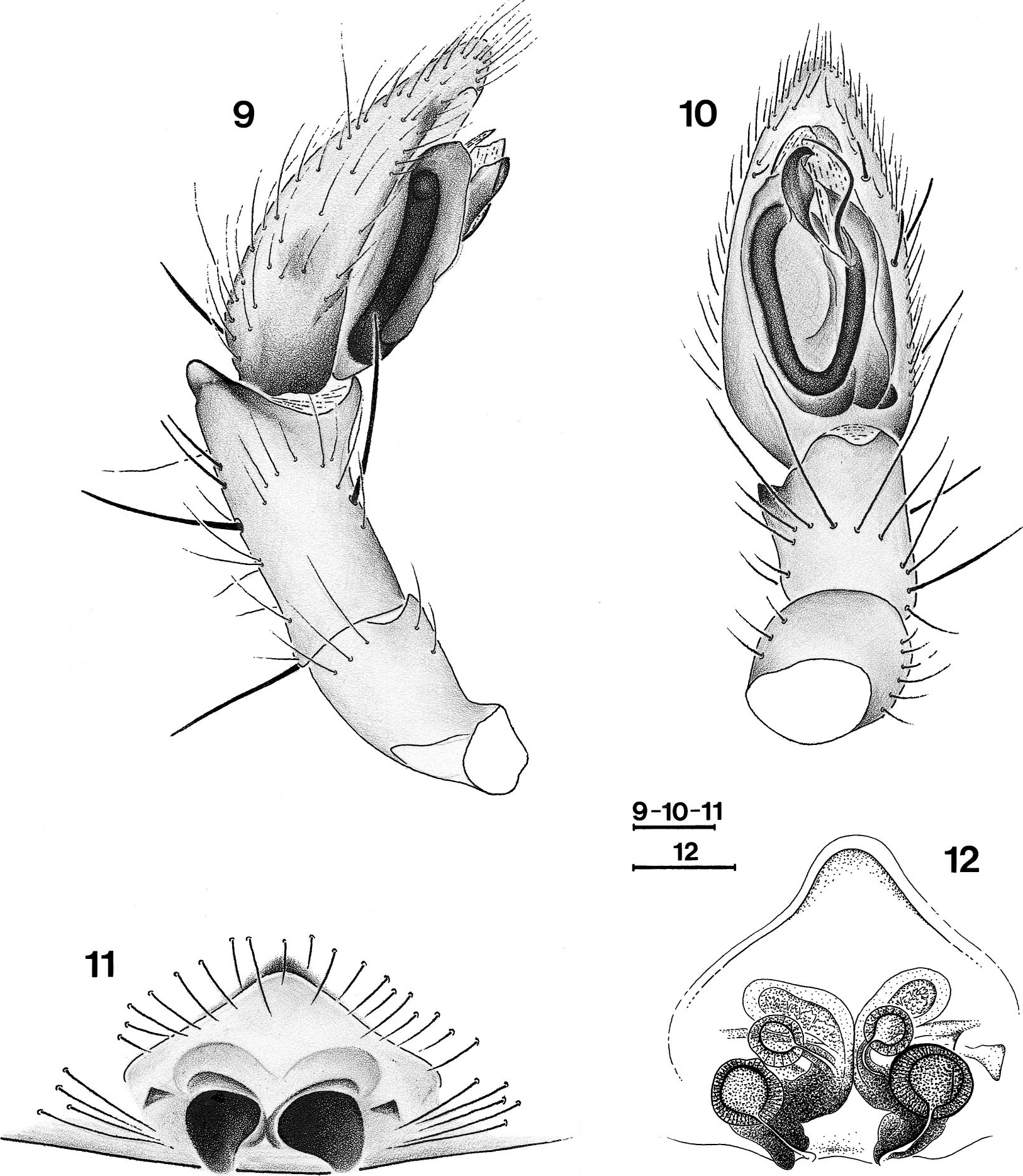
Diagnosis. —Specirnens of Toxoniella have far more spines than representatives of Madagascan Gallieniellidae . Male representatives of Toxonlella have an oval tegulum with a posterior extension but lack a tegular central ridge; the embolus as well as the median apophysis and the embolar membrane are short and simple; females are characterized by the epigyne with long cul de sac tubes in front of the spermathecae which are double, each pair consisting of two well separated spheres.
Etymology. —The name is derived from the Greek τοξου which means arch, and refers to the presence of the taxon in the Eastern Arc mountains. The gender is feminine.
Natural history. —All specimens were caught in mountain forest by pitfall traps, sieving litter or hand collecting. Some of these forests are tiny remnants not exceeding a few ha. The elevation distribution ranges from 400-1200 m.
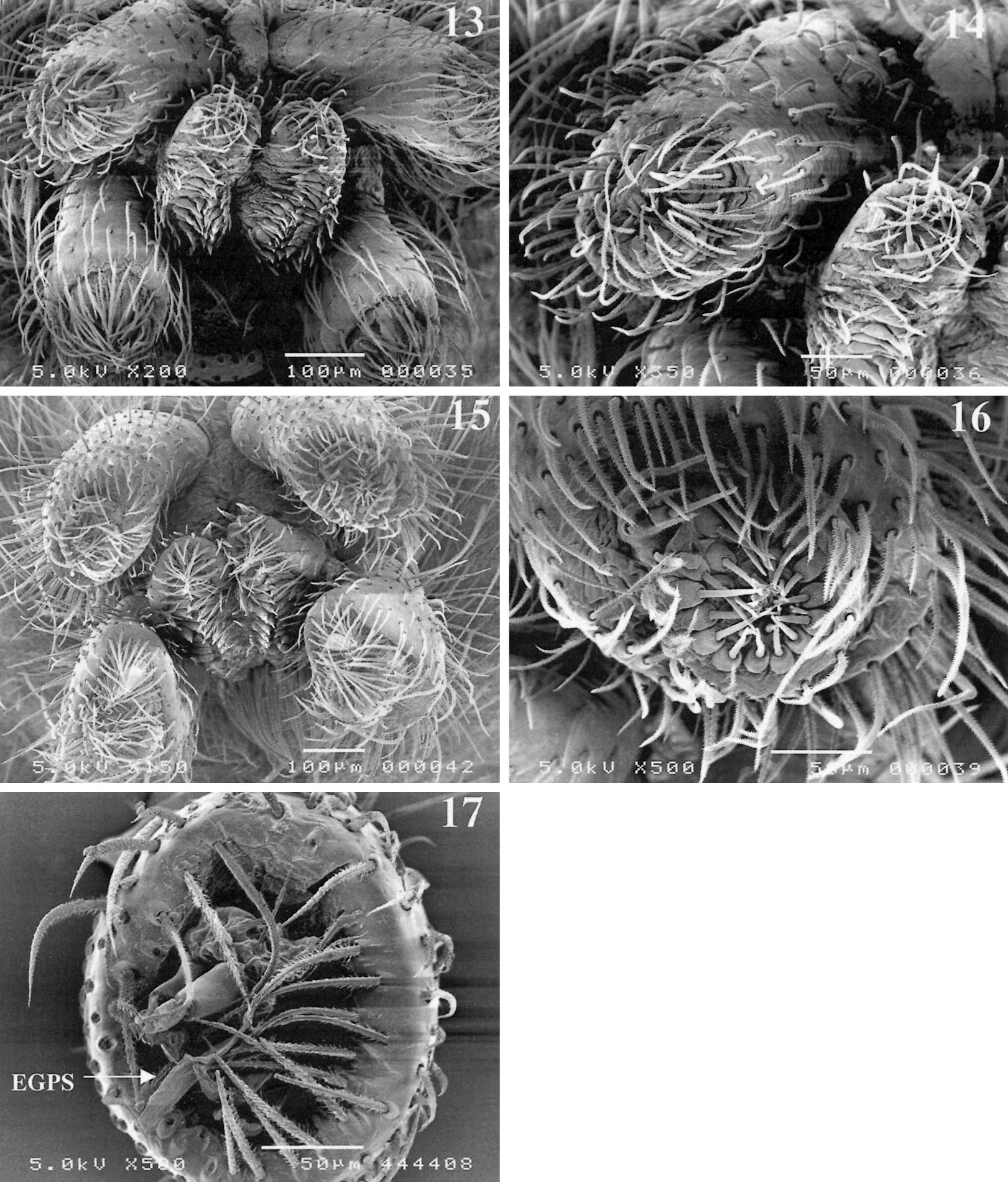
Affinities. — The position of Toxoniella is problematic in that the females fit the Gallieniellidae (absence of EPGS) whereas the males should be placed in the Gnaphosidae as they possess these typical spigots. However, the Gallieniellidae have thus far only been defined ( Platnick 1990) by the absence of EPGS piriform gland spigots and the presence of a distal sclerotized ring on the ALS, both plesiomorphic characters. In the absence of a sound definition of the Gallieniellidae there are two possibilities for the placement of Toxoniella both of which imply that they are in fact intermediate between the Gallieniellidae and the Gnaphosidae . The genus can either be regard ed as a derived gallieniellid in which only the males have acquired EPGS or as an ancestral gnaphosid in which the females have not yet acquired EGPS and retained a distal sclerotized ring in females. A third possibility exists that would consider Toxonlella a derived gnaphosid in which the EGPS have reversed into a distal sclerotized ring in females. The latter possibility is difficult to maintain for two reasons. The reversal of the EPGS into a previously lost sclerite is a most unlikely evolutionary step and the genus is apparently related to the South African Drassodella in which both sexes lack EPGS. This relationship is the main argument to accommodate Toxoniella among the gallieniellids. These genera share the dense spination that is absent in the Madagascan members of the family, rows of lamelliform hairs under the tarsal claws ( Figs. 18-21 View Figures 18 - 21 ), a pair of prolateral abdominal sigilla (see figs. 32, 33 in Jocque 1999) and frontal cul de sac expansions in the epigyne ( Figs. 8 View Figures 4 - 8 , 12 View Figures 9 - 12 ). In Drassodella these are bladder-like whereas they are clearly longer than wide in Toxoniella . Both the genera further possess a posterior extension of the tegulum, not connected with the origin of the embolus as in Gallieniella . The main differences in the pair of African continental genera is the absence of a central tegular ridge in Toxoniella , present in Drassodella and pairs of well separated spermathecae present in the former, absent in the latter, where the spermathecae appear to be constricted.
Description. —Small to medium -sized spi ders (3-9) with oval carapace, widest between coxae II and III; narrowed in front to about 0.65 times maximum width. In profile rather flat, thoracic area lower than cephalic one, highest point just behind posterior eyes. Cervical grooves poorly indicated. Color: prosoma, including chelicerae and legs yellowish brown, covered with short, brownish golden setae; abdomen gray with dense cover of brownish golden setae. Eyes in two recurved rows, subcircular and subequal, except PME smaller oval and flat. Clypeus low, slightly more than diameter of ALE, straight with few setae. Chilum single, triangular. Chelicerae only slightly prolonged, extending forward about one fifth of carapace length (the variable individual inclination makes this figure not very relevant). Endites fairly broad, smoothly constricted opposite insertion of trochanters. Sternum shield-shaped with dispersed setae; coxae IV narrowly separated. Labium slightly longer than broad; hardly widened at base. Legs: formula 4123. Spination: fewer spines on anterior leg pairs than on posterior pairs. TI and TII, sometimes PI and PII, in male with ventral rows of long recurved setae. Mt III and IV with poorly developed preening brush. Claws with about 3-7 teeth, more numerous on anterior legs. With two rows of up to 6 lamelliform setae under claws ( Figs. 20, 21 View Figures 18 - 21 ). No scopulae. Abdomen oval, without scutum in both sexes; frontal part of male abdomen slightly sclerotized. Four dorsal sigilla and one small lateral one on either side. Six spinnerets: ALS in females with sclerotized subdistal ring, slightly conical, closely set; piriform gland spigots well developed but not enlarged ( Figs. 15, 16 View Figures 13 - 17 ); males with some EPGS ( Fig. 17 View Figures 13 - 17 ), without sclerotized distal ring. Male palp: tibia with small dorsolateral apophysis. Tegulum with posterior extension, not containing the sperm duct and frontal tapered extension. Embolus, median apophysis and embolar membrane all short and simple. Epigyne with single, wide, frontal ledge and curved lateral grooves; entrance ducts short but with frontal cul de sac tubes in front of double spermathecae.
Distribution. — Only known from the Taita Hills, southeastern Kenya.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.