Trachipterus Goüan 1770
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5039.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:234D03A3-1AC7-442E-A8A5-784EB3EE4394 |
|
persistent identifier |
https://treatment.plazi.org/id/03E29102-FF90-FF98-C78F-31934A3CAAA9 |
|
treatment provided by |
Plazi |
|
scientific name |
Trachipterus Goüan 1770 |
| status |
|
Trachipterus Goüan 1770 View in CoL View at ENA
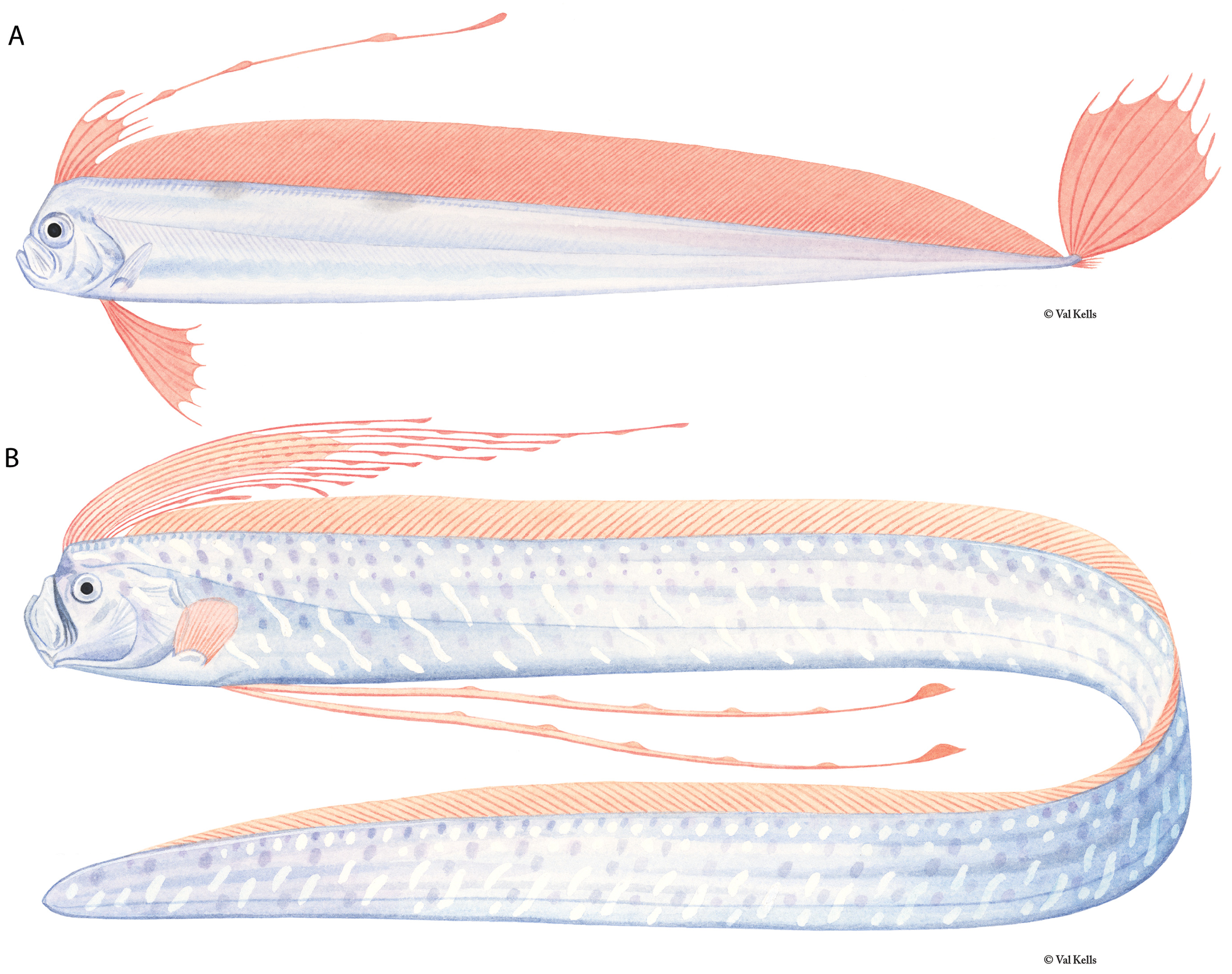
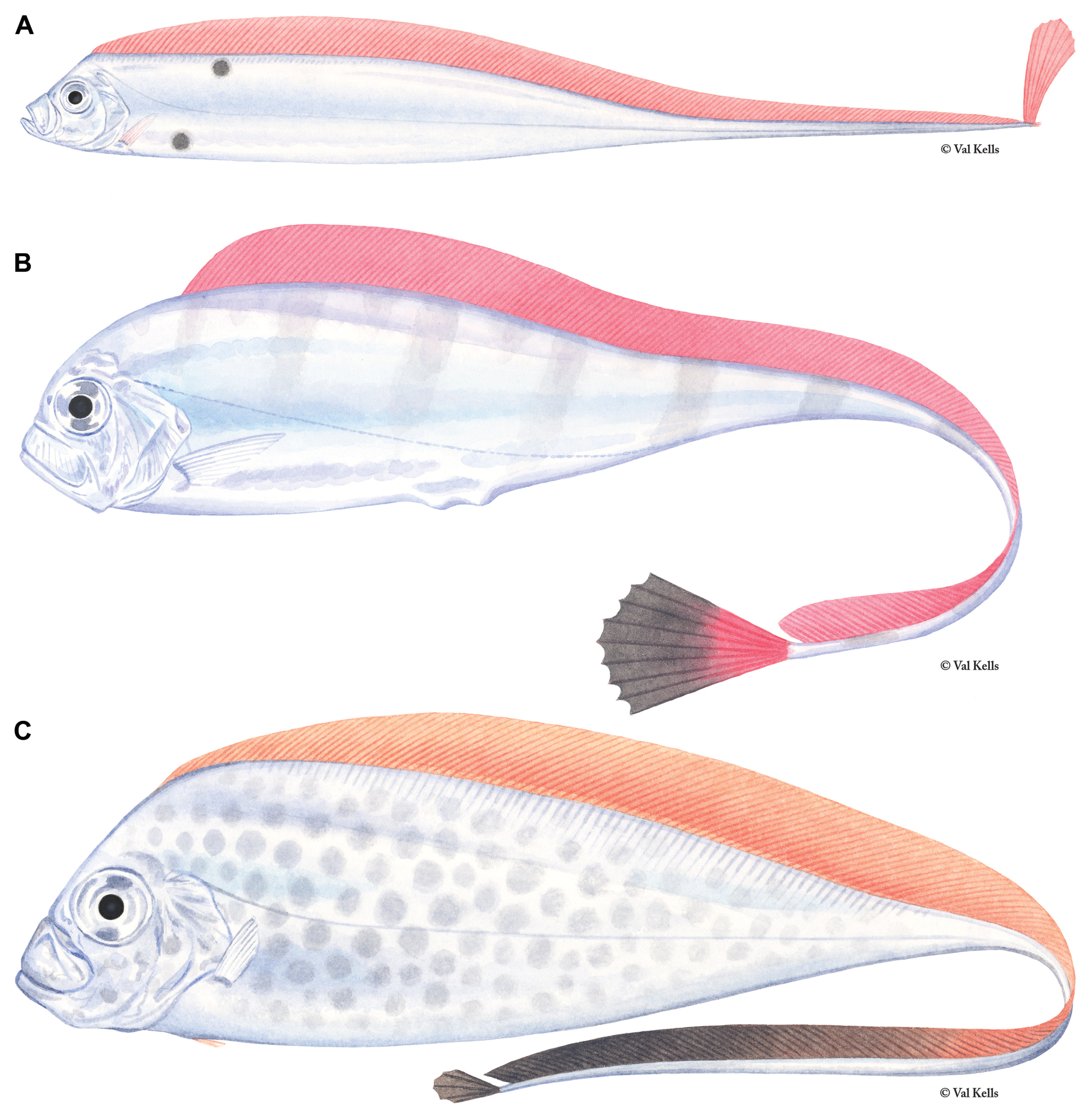
Figures 1A View FIGURE 1 , 2A View FIGURE 2 , 3A View FIGURE 3 , 4–7 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 , 9–17 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17
Type species. Cepola trachyptera Gmelin 1789 , by subsequent designation; genus first described without a type species.
Trachipterus Goüan 1770 View in CoL . Hist. Pisc. p. 104 ( Cepola trachypera Gmelin )
Gymnogaster Brünnich 1788 View in CoL . K. Dansk. Vid Selsk. p. 408 ( arcticus View in CoL )
Trachyterus Bloch & Schneider 1801 . Blochii Syst. Ichth. p. 480 ( taenia View in CoL )
Bogmarus Bloch & Schneider 1801. Blochii Syst. Ichth. p. 518 (islandicus) [name only]
Argycticus Rafinesque 1810. Caratt. Nuov. Gen. p. 55 (quadrimaculatus)
Cephalepis Rafinesque 1810 . Ind. Ittiol. Siciliana. p. 54 (octomaculatus)
Trachypterus Cuvier 1816 . Le Règne Animal.
Epidesmus Ranzani 1818 . Opusc. Sci. Bologna. p. 137 (maculatus)
Material Examined. Here we list holotypes of species of Trachipterus that were examined in this study, as well as all other Trachipterus specimens examined. However, the latter are all listed as Trachipterus spp. pending a specieslevel revision of the genus, which is beyond the scope of this paper.
Holotypes examined: Trachipterus arawatae Clarke 1881 , NMNZ P.1008 (Holotype, 551 mm SL, Pacific, New Zealand, South Island, Westland); Trachipterus ishikawae Jordan & Snyder 1901, NSMT-P 589 (Holotype, 1250 mm SL, Pacific, Japan, off mouth of Tokyo Bay); Trachipterus jacksonensis ( Ramsay 1881) , AMS A.9114 (Holotype, 736 mm SVL, Pacific, Australia, New South Wales, Manly Beach); Trachipterus gryphurus Lowe 1852 , BMNH 1917.7.14.83 (Holotype, radiograph, 635 mm SL, Atlantic, Portugal, Madeira Islands); Trachipterus pentastigma Norman 1922 , BMNH 1922.6.7.48 (Holotype, radiograph, 121.5 mm SL, Pacific, Japan); Trachypterus rexsalmonorum Jordan & Gilbert 1894, CAS: SU ( ICH): 1060 (Holotype, radiograph only, 284 mm SL, Pacific, California, off San Francisco).
Other specimens of Trachipterus spp. examined. Because of the uncertain alpha taxonomy of the genus Trachipterus , which is beyond the scope of this paper, we list all specimens as spp., although many are cataloged as species based on distribution. AMS IB.6691 (1060 mm SL, Pacific, Australia, New South Wales); AMS I.17712 (Dry specimen, 1400 mm TL, Pacific, Australia, New South Wales, Port Jackson ) ; AMS I.21367-035 (165 mm SL, Pacific, Australia, New South Wales, Newcastle ) ; AMS I.24575-001 (Radiograph, 30 mm SL, Pacific, Australia, Queensland, Coral Sea ) ; AMS I.24159-001 (Radiograph, 35 mm SL, Pacific, Australia, New South Wales, Jervis Bay ) ; AMS I.25640-001 (1440 mm SL, Pacific, Australia, New South Wales, off Sydney ) ; AMS I.32117.001 (1860 mm SL, Pacific, Australia, New South Wales, Crowdy Bay ) ; AMS I.36212-001 (1800 mm SL, Pacific , Australia, New South Wales , BMNH 2010.3.23.16–17 (2 specimens, 32.7–59.5 mm SL, Atlantic, off northwest Africa ) ; BMNH 2010.3.23.21–26 (6 specimens, radiographs, 50–120.3 mm SL, Atlantic, King’s Trough Flank ) ; CSIRO H1536-1 View Materials (1454 mm SL, Pacific , Tasmania, southwest of Tasmania) ; CSIRO H241 View Materials (1100 mm SL, Pacific, Tasman Sea, southeast Tasmania, east of Maria Island ) ; CSIRO H243 View Materials (471 mm SVL (1089 mm TL), Pacific, Tasman Sea, Cascade Plateau ) ; CSIRO H245 View Materials (1370 mm SL, Pacific, Tasman Sea, southeast Tasmania, east of Maria Island ) ; CSIRO H932-1 View Materials (Radiograph, 237.4 mm SL, Pacific, Tasman Sea, southeast Tasmania, Maria Island ) ; CSIRO 2036 View Materials (Radiograph, 204.01 mm SL, Pacific, Tasman Sea, Fortescue Bay ) ; CSIRO 2037 View Materials (151.24 mm SL, Pacific, New Zealand, Cook Strait ) ; CSIRO 3859-01 View Materials (Radiograph, 980 mm SL, Pacific, northwestern Tasmania, Bass Strait ) ; CSIRO B3912 View Materials (Radiograph, 141.1 mm SL, Pacific, Tasman Sea, southeast Tasmania, southeast of Maria Island ) CSIRO H4947 View Materials (183.32 mm SVL, Pacific, Tasman Sea, South Tasman Rise ) ; CSIRO H6328 View Materials (1970 mm SL, Pacific, western Tasmania, west of Granville Harbour ) ; CSIRO unregistered GT 1160 (Frozen, 944 mm SL, Pacific, southern Tasmania, off Maatsuyker Island ) ; CSIRO unregistered (Frozen, 185 mm SL, Pacific, Tasman Sea, eastern Tasmania, Adventure Bay ) ; HUMZ 69219 View Materials (1346 mm SL, Pacific , Japan, Hokkaido) ; HUMZ 80914 View Materials (1209 mm SL, Pacific, central Pacific ) ; HUMZ 132216 View Materials (239 mm SL, unknown) ; HUMZ 141393 View Materials (55.9 mm SL, Pacific , Japan) ; HUMZ unregistered (630 SVL, unknown); KPM-NI0007802 About KPM-NI (254 mm SL, Pacific , Japan, Shizuoka Prefecture) ; KPM- NI0010429 (2472 mm SL, Pacific , Japan, Shizuoka Prefecture) ; KPM-NI0011445 About KPM-NI (2240 mm SL, Pacific , Japan, Tokyo) ; KPM-NI0011644 About KPM-NI (2350 mm SL, Pacific , Japan, Kanagawa Prefecture) ; KPM-NI0012738 About KPM-NI (2114 mm SL, Pacific , Japan, Shizuoka Prefecture) ; KPM-NI0012764 About KPM-NI (2472 mm SL, Pacific , Japan, Kanagawa Prefecture) ; KPM- NI0012765 (1889 mm SL, Pacific , Japan, Aichi Prefecture) ; KPM-NI0012766 About KPM-NI (2152 mm SL, Pacific , Japan, Aichi Prefecture) ; KPM-NI0013001 About KPM-NI (1680 mm SL, Pacific , Japan, Shizuoka Prefecture) ; KPM-NI0013233 About KPM-NI (1960 mm SL, Pacific , Japan, Kanagawa Prefecture) ; KPM-NI0016297 About KPM-NI (285 mm SL, Pacific , Japan, Chiba Prefecture) ; KPM- NI0017321 (2000 mm SL, Pacific , Japan, Aichi Prefecture) ; KPM-NI0023327 About KPM-NI (215 mm SL, Pacific , Japan, Akita Prefecture) ; KPM-NI0023505 About KPM-NI (21 mm SL, Pacific , Japan, Shizuoka Prefecture) ; KPM-NI0025081 About KPM-NI (1880 mm SL, Pacific , Japan, Kanagawa Prefecture) ; KPM-NI0027573 About KPM-NI (61 mm SL, Pacific , Japan, Kanagawa Prefecture) ; MCZ 3895 About MCZ (8 specimens, radiographs (of the largest), 19–230 mm SL, Atlantic, Mediterranean, off Sicily ) ; MCZ 8644 About MCZ (≈ 184 mm SL, Atlantic, northeastern, Portugal, Azores, Fayal ) ; MCZ 8645 About MCZ (2 specimens, radiographs, 160–213 mm SL, Atlantic, northeastern, Portugal, Azores, Fayal ) ; MCZ 58926 (4 specimens, 17–32.5 mm SL, Atlantic, eastern central Atlantic ) ; MCZ 58958 (11 mm SL, Atlantic, western central Atlantic ) ; MCZ 58959 (16 mm SL, Atlantic, western central Atlantic ) ; MCZ 135299 About MCZ (Radiograph, 1535 mm SL; no collection data) ; MCZ 143323 About MCZ (estimated at 1000 mm SL, head only, Atlantic, northwestern, off Delaware ) ; MCZ 147873 About MCZ (8 specimens, radiographs (6), 21–61 mm SL, Atlantic, Mediterranean, off Naples ) ; MCZ 163198 About MCZ (Radiograph, ≈ 415 mm SL, Atlantic, northwestern, Bear Seamount ) ; NMI 63.1937 View Materials (509 mm SL, Atlantic , Ireland, Donegal) ; NMI 66.1994 View Materials (305 mm SL, Atlantic , Ireland) ; NMNZ P.001961 (249 mm SL, Pacific, New Zealand, Stewart Island ) ; NMNZ P.002056 (cleared and stained, 432 mm SL, Pacific, New Zealand, South Island , Marlborough) ; NMNZ P.007087 (89.7 mm SL, Pacific, New Zealand, North Island , Wellington) ; NMNZ P.016410 (495 mm SL, Pacific, New Zealand, Kermedec Trench / Louisville Ridge ) ; NMNZ P.016453 (1880 mm SL, Pacific, New Zealand, North Island, South Auckland) ; NMNZ P.031676 (603 mm SL, Pacific, New Zealand, South Island , Marlborough) ; NMNZ P.037649 (Radiograph only, 960 mm SL; Pacific New Zealand, South Island , Nelson) ; NMNZ P.037650 (Radiograph only, 770 mm SL; Pacific New Zealand, South Island , Marlborough) ; NMNZ P.037894 (183 mm SL, Pacific, New Zealand, South Island , Otago) ; NMNZ P.041259 (564 mm SL, Pacific, New Zealand, South Island , Marlborough) ; NMNZ P.041957 (2023 mm SL, Pacific, New Zealand, North Island , Taranaki) ; NMNZ P. 041970 (Radiograph, 1724 mm SL, Pacific, New Zealand, North Island, Karikari Peninsula ) ; NMNZ P.042021 (797 mm SL, Pacific, New Zealand, South Island, Parapara ) ; NSMT- P 12367 (604 mm SL, Pacific, Japan, Suruga Bay ) ; NSMT-P 40508 (255 mm SL, Atlantic , Suriname) ; NSMT-P 57538 (101.9 mm SL, Pacific, western North Pacific ) ; NSMT-P 57554 (6 specimens, 45–115 mm SL, Pacific, western North Pacific ) ; NSMT-P 57560 (13 specimens, 105–220 mm SL, Pacific, western North Pacific ) ; NSMT-P 57670 (4 specimens, 20–155.9 mm SL, Pacific, western North Pacific ) ; NSMT-P 76536 (80 mm SL, Pacific, Japan, Ryukyu Islands ) ; QVM 1971.5.3 (75 mm SVL, Pacific, Tasman Sea, eastern Tasmania ) ; QVM 1973.5 View Materials .36 (212 mm SL, Pacific, northern Tasmania, Bass Strait ) ; VIMS 25015 View Materials (1205 mm SL; Atlantic, Charlie Gibbs Fracture Zone ) ; VIMS 42302 View Materials (est. 1000 mm SL; Atlantic, Charlie Gibbs Fracture Zone ) ; NMP P6 View Materials V 5066 (Dry specimen, 1500 mm, Atlantic, Mediterranean, France, Marseille ) .
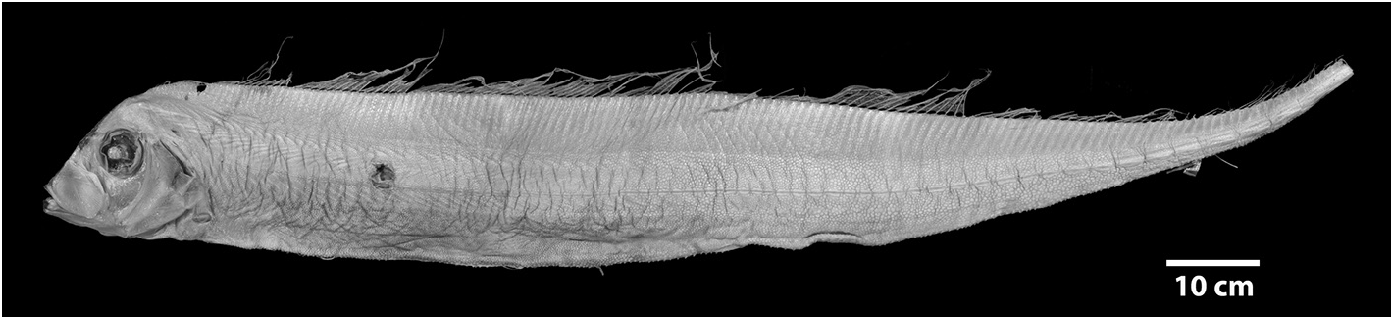
Diagnosis (Adults). Body long (to 2640 mm SL), laterally compressed (more compressed than Zu or Desmodema ) tapering to a thin caudal peduncle, not greatly constricted posterior to the vent ( Fig. 3 View FIGURE 3 ). Ventral edge of body nearly straight with pointed tubercles. Dermal tubercles and pore system present throughout trunk. Scales cycloid and deciduous, covering the body (commonly overlooked). Lateral line dropping to mid-body on trunk just posterior to the pectoral fins, continues well above the ventral edge in the tail region until the base of the caudal fin; lateralline plates armed with 1 (rarely 2) subconical spines, typically with one peak (rarely 2), spines most prominent in caudal region of larger adults. Relative to the lateral-line scale, spines angled anteriorly (not pointed laterally as in Desmodema or Zu). Body depth at pectoral fin 3.3–4.5 in SV. Premaxilla with 5–20 strong caniniform teeth, 5–27 teeth on dentary; vomer with 1–4 (most often 1–2) strong teeth; palatine teeth either absent or up to 3 (at least one Trachipterus sp. with 12–15 teeth on each palatine). Gill rakers on the first arch 2-4 + I +7-10, all with multiple spinules. Pseudobranch well developed. Branchiostegal rays 6. Dorsal fin originating from ½ eye diameter to posterior margin of eye. Dorsal-fin rays 133–194, first 4–7 (typically 5 or 6) of which are stout; rays evenly spaced (typically broken in adults); interspace present, remaining fin rays filamentous. Pectoral-fin with one exceptionally short fin ray followed by 8–16 rays (1+ 8-16); short fin ray typically decreasing in length with increasing SL; rarely fused with second ray. Pelvic fins appear absent in adults, 4–9 fin-rays decrease in length as SL increases until only a slitlike opening is apparent (as in adult Zu, never healed as in Desmodema ). Anal fin absent. Caudal fin in two parts; dorsal lobe set at steep, anterior-facing angle to the caudal peduncle, with 8–9 rays (rarely 10), two outermost rays thicker than innermost rays; ventral lobe with up to 6 rays, all greatly reduced with the bases remaining as rudimentary spine-like elements. Minute spinules on the dorsal, pectoral, and caudal-fin rays greatly reduced or absent.Anus located on midventral line (rarely on the left side). Total vertebrae 69–102.
Color (adults). Body silver, occasionally with a dark patch spread across the ventral region anterior to the anus; dentary and premaxilla black in frontal view. Dorsal-fin rays bright red or crimson, dorsal midline black; caudal fin black (red through at least large juveniles).
Remarks. Most descriptions of the genus Trachipterus have primarily relied on data from juvenile specimens. True adult specimens (vs. large juveniles) have rarely been examined and reported in the literature. Savinykh & Baitalyuk (2011) completed a limited morphological examination of 20 large specimens of Trachipterus from the northern Pacific Ocean. These authors list a maximum size for the genus as 2900 mm SL (this is mistakenly listed as 290 mm, rather than cm, in Savinykh & Baitalyuk 2011: table 3) but only report meristic and morphometric data for specimens ranging in size from 910–1790 mm SL. They assumed all individuals examined had undergone metamorphosis, a process that is considered herein to be very protracted and not directly determined by body size (see below). The authors do not report any information regarding pelvic fins, a character that changes drastically throughout ontogeny, and therefore developmental stages of these specimens cannot be inferred. The present study is the first account to include data from large adult specimens (> 1790 mm SL, N=16) in a revised description of the genus and provides the first synthesized account of the biology and habitat of large adult Trachipterus .
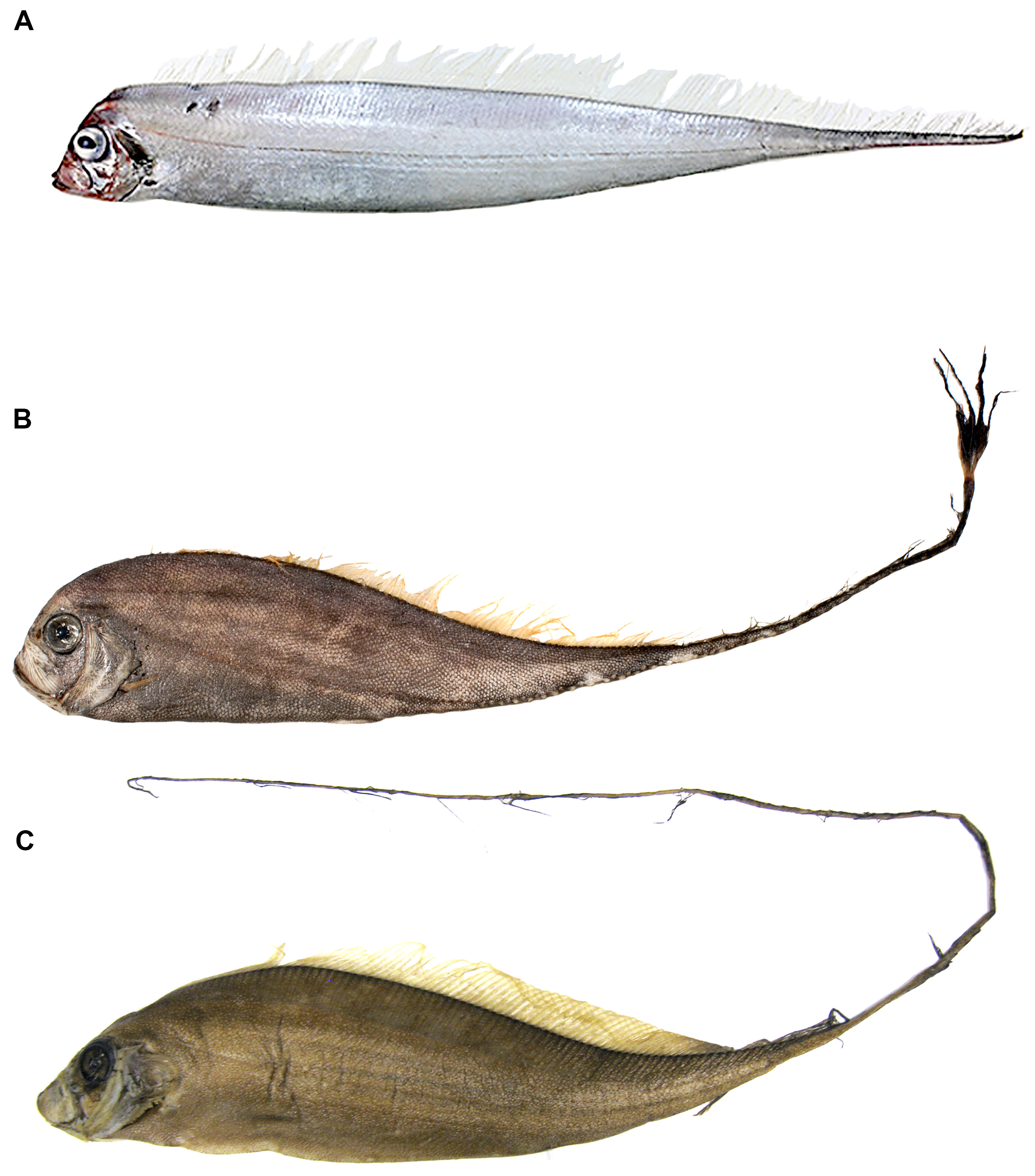
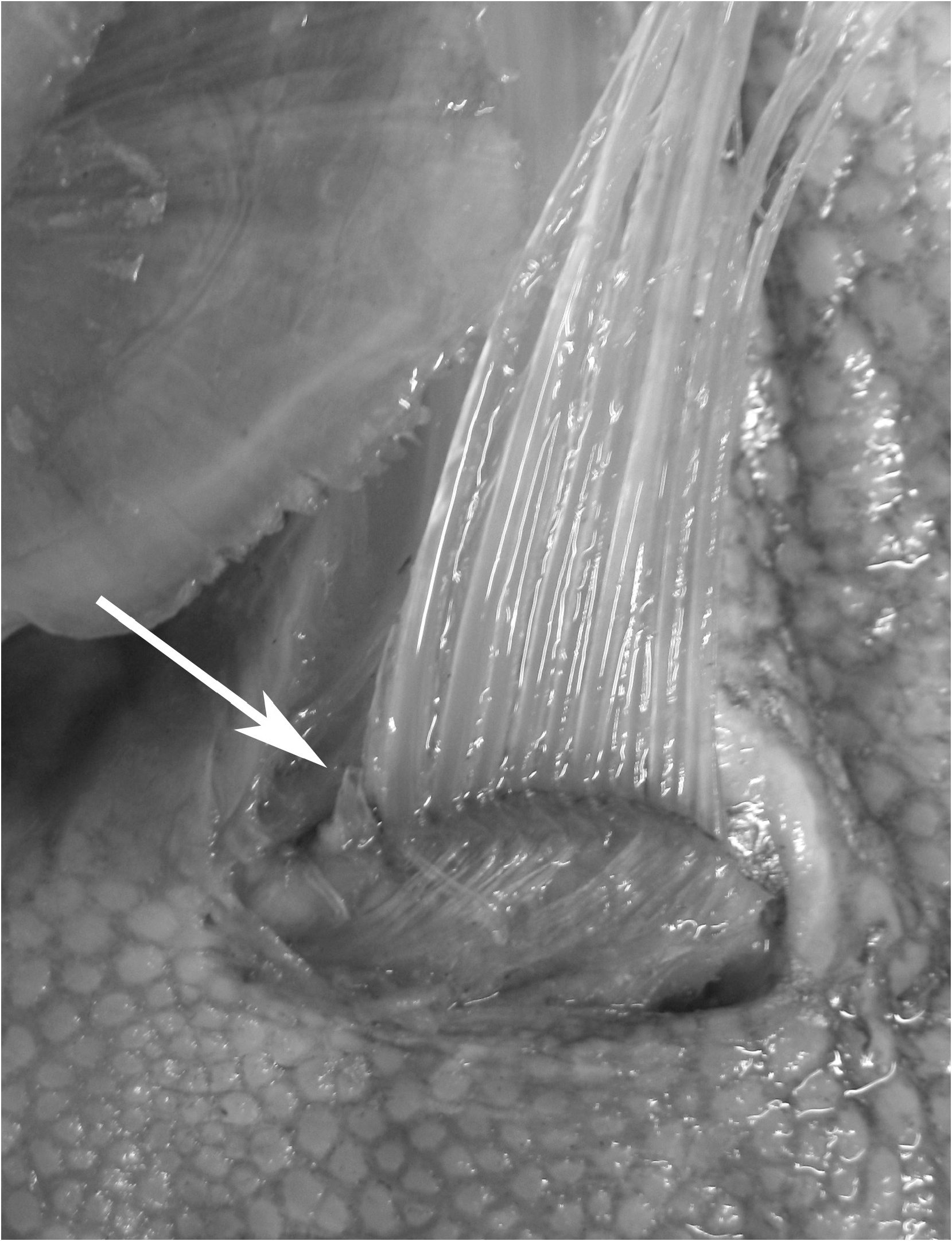
Examination of larger specimens than in previous studies has also allowed for more accurate descriptions of the ontogeny of the pelvic fin (discussed further in Ontogeny: juvenile to adult). In large adult specimens, pelvic-fin rays are completely reduced to the bases, with no shortened pelvic-fin rays, or stubs, as present in larger juveniles. As with the largest specimens of Zu, a slit remaining at the pelvic-fin origin is the only remnant of the elongate pelvic-fin rays present in juveniles ( Fig. 4 View FIGURE 4 ). To date, no specimens of Trachipteru s examined have the pelvic scar completely healed over, as found in Desmodema .
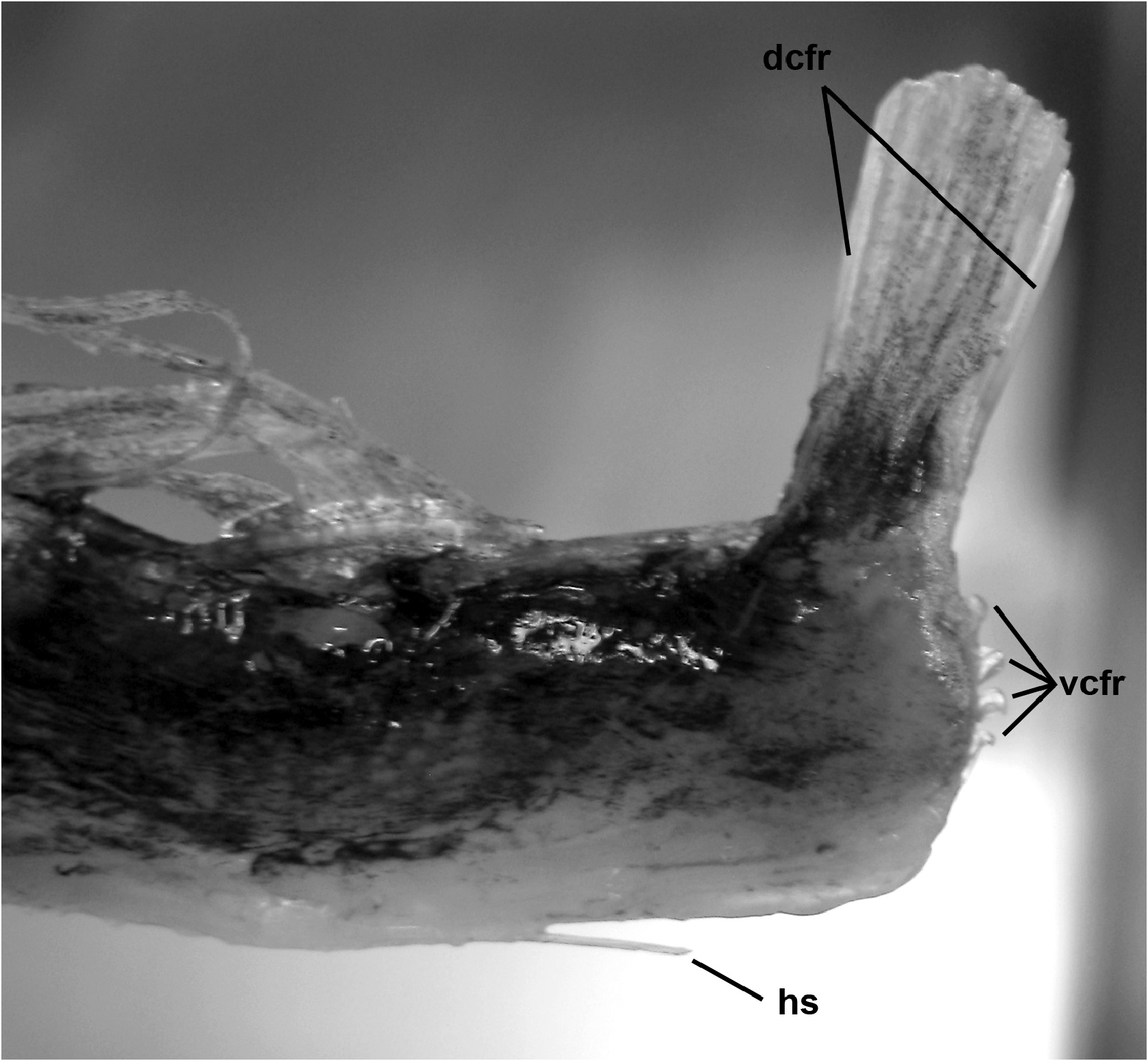
Pectoral-fin ray counts previously reported for Trachipterus range from 8–14. Specimens examined in this study greater than 50 mm SL all show the pectoral fin consisting of one short, spine-like element ( Fig. 5 View FIGURE 5 ) followed by 9–16 longer fin rays, here notated as 1+9–16. It is unclear if most previous authors included the short element as part of the total pectoral-fin-ray count as, in some specimens, this element can be easily overlooked. One specimen (AMS IB.6691 645 mm SV) has a pectoral fin-ray count of 1+16 (left) and 1+15 (right), counts not previously reported in the literature for Trachipterus .
Roberts (2012) referred to figures of a so-called “accessory caudal fin” in juvenile and adult specimens of Trachipterus with fin rays extending distally and ventrally and suggested that this structure might be an anal fin. The absence of an anal fin, in part, defines the family Trachipteridae , and is a synapomorphy uniting the Trachipteridae + Regalecidae clade ( Olney et al. 1993). No citation was given for this structure and it is likely to be one of two things: 1) the reduced rays on the ventral caudal lobe in Trachipterus (as in McCoy 1886, plate 122, fig. 2; Hamilton 1916); or 2) haemal spines that sometimes pierce the ventral body wall in the posterior portion of the tail region. This latter condition is not uncommon in Desmodema and had been mistakenly referred to as an anal fin (see Desmodema , this study). It is seen occasionally in large Trachipterus (KPM 25081 1880 mm SL; Fig. 6 View FIGURE 6 ), but not to the extent it is present in Desmodema . This is possibly due to greater dorsoventral constriction in the posterior tail region of Desmodema . No trachipterid specimens examined in this study at any ontogenetic stage possess an anal fin.
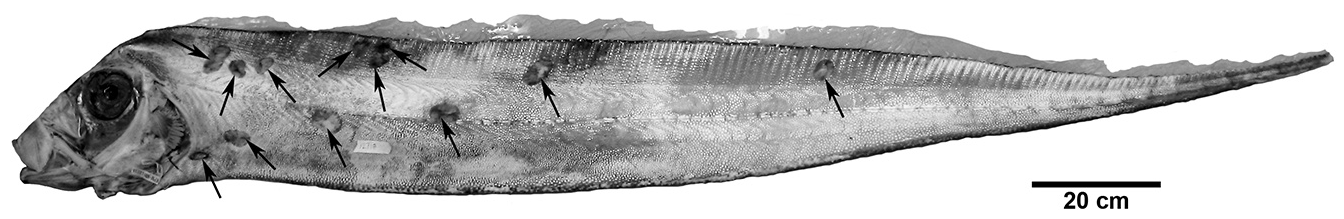
It is not uncommon for large specimens (> 1600 mm) to have scars resulting from what appear to be bites of cookie-cutter sharks, Isistius spp. In this study, 56% of large specimens had one or more scars matching the wound marks left by Isistius spp. As many as 22 scars have been found on a single Trachipterus specimen (KPM 12738, 2114mm SL; Fig. 7 View FIGURE 7 ). Mincarone et al. (2001) reported on three large (1670–1860 mm SL) western South Atlantic specimens of Trachipterus sp. from southern Brazil of which all had both recent and healed cookie-cutter shark wounds. Roberts (2012) mistakenly referred to a Lophotus from New South Wales, Australia (AMS I.43718) as the “only well-documented example of a lampridiform fish bitten by a cookie-cutter shark”. However, most larger specimens of lampridiform fishes examined in the present study (e.g., Trachipterus , Regalecus , and Lophotus ; JMM, pers. obs.) have similar bite scars.
Morphology of juveniles. Nearly all nominal species in Trachipterus ( Table 1) are based on juvenile specimens, as this life stage is present in shallower, nearshore waters and are better represented in systematic collections (when compared to adults). Like adults, juveniles are laterally compressed, but numerous other morphological differences exist. Due to allometric growth and morphological changes throughout ontogeny, different life-history stages of juvenile and adult specimens of the same species can appear drastically different in regards to: 1) general body shape; 2) fin length and number; and 3) pigmentation patterns.
Relative to the standard length, juvenile Trachipterus spp. have a greater head and snout-vent length and are deeper bodied than adults and, conversely, tail length (vent to caudal fin) is relatively shorter in juveniles ( Scott 1983, fig. 1; Scott 1984, fig. 1; Fig. 8 View FIGURE 8 ). Lateral-line orientation also varies between the two stages. In the posterior half of the tail in juvenile Trachipterus , the lateral line runs close to the ventral margin versus its mid-body position in adults.
In juveniles, the first 5–6 dorsal-fin rays are typically elongate. There is a membranous interspace between these anteriormost rays and the more posterior portion of the dorsal fin (i.e., the majority of the fin), which is all that is present in adults. In several specimens examined in this study, however, the condition is slightly different and consists of 1 short fin ray, followed by 4–5 elongate rays that successively decrease in length and are followed by a space (e.g., NSMT 57670, 20 mm SL; KPM 27573, 61 mm SL ( Fig. 9 View FIGURE 9 ); HUMZ 132216, 239 mm SL). Having the first ray being much shorter than the remaining rays is also apparent in the pectoral and pelvic fins. Observing a small juvenile specimen (12.9 cm SL) that was dip-netted near the surface and maintained in an aquarium, Moritz et al. (2015) described potential luring behavior, in which the elongate dorsal-fin rays were moved in a wide arc approaching 180°.
Pelvic-fin rays in early juveniles are greatly elongate ( Fig. 9 View FIGURE 9 ) and the fin consists of 0–1 + 5–9 fan-like rays that have bulb-like swellings present (typically only seen in live specimens); the first elongate ray is more stout than the others. The first short, spine-like ray may be absent and is easily overlooked when it is present. The length of pelvicfin rays can reach well beyond the tip of the caudal fin in young specimens. Two lobes are present in the caudal fin. The dorsal lobe contains 6–10 elongate rays, fan-like in life and is held vertically in live individuals ( Fig. 2A View FIGURE 2 ) with the two outermost rays thicker than the inner rays ( Fig. 10 View FIGURE 10 ). The ventral caudal lobe contains 4–7 rays that are not as reduced as they are in adults ( Fig. 6 View FIGURE 6 ), but are rather represented as short, spine-like bases.
Juvenile Trachipterus trachypterus were observed in situ by Macali et al. (2020) in the upper 10 m of water during nighttime SCUBA dives near an upwelling on the Mediterranean coast of Italy. These individuals were observed to orient vertically, and upon recognition of potential predators (the divers), the fish would orient with their heads up and their elongate pelvic and caudal-fin rays extended. Periodic, quick flicking of these fins was hypothesized by Macali et al. (2020) to mimic jellyfishes. From video from a submersible, Mortiz et al. (2015) also observed a juvenile maintaining a vertical orientation and using undulation of its dorsal fin for locomotion.
A juvenile Trachipterus (KPM 27573, 61 mm SL; Fig. 9 View FIGURE 9 ), that was dip-netted from the Sea of Japan, kept alive for 12 hours and immediately frozen at death, displayed minimal damage. This specimen, which was the most complete available for this study, was defrosted and examined immediately. Because of the condition of the of the fish, numerous morphological characters not previously observed, likely due to capture damage of these extremely fragile fishes, could be described and are presented below.
The presence of lateral spinules on the dorsal-, caudal-, and pelvic-fin rays are characters shared by all trachipterid fishes. However, in KPM 27573 the spinules on the first elongate dorsal-fin ray are not laterally directed, but rather project anteriorly. McCoy (1886: plate 22.1) describes and illustrates a similar condition of the pelvic fins, in which the first pelvic-fin ray has “a row of spinular granules on front”. This condition was not observed in any other trachipterid specimens examined in this study and its preservation is attributed to specimen quality in both cases, as McCoy’s (1886) specimen was hand-delivered shortly after capture and examined when it was freshly dead. The anterior orientation in these spinules corresponds with the vertical orientation that is assumed by trachipterid fishes and possibly adds a level of protective value, as the preceding element is extremely short, or it could serve a hydrodynamic function.
Branching of fin rays has been reported in the posterior-most rays of the pectoral and pelvic fins ( McCoy 1886; Jordan & Gilbert 1894). However, all fins of KPM 27573 possessed branched rays: the posteriormost fin rays of the pectoral and pelvic fins, the inner-most caudal-fin rays, and the anteriormost elongate dorsal-fin rays. As these regions of the pectoral, pelvic, and caudal are the most fragile and filamentous of the fin-rays, it is likely that the most distal, branching ends of these rays typically are damaged or lost during capture or preservation. Branching of the pelvic fins was also observed in specimens up to 604 mm SL (NSMT 12367).
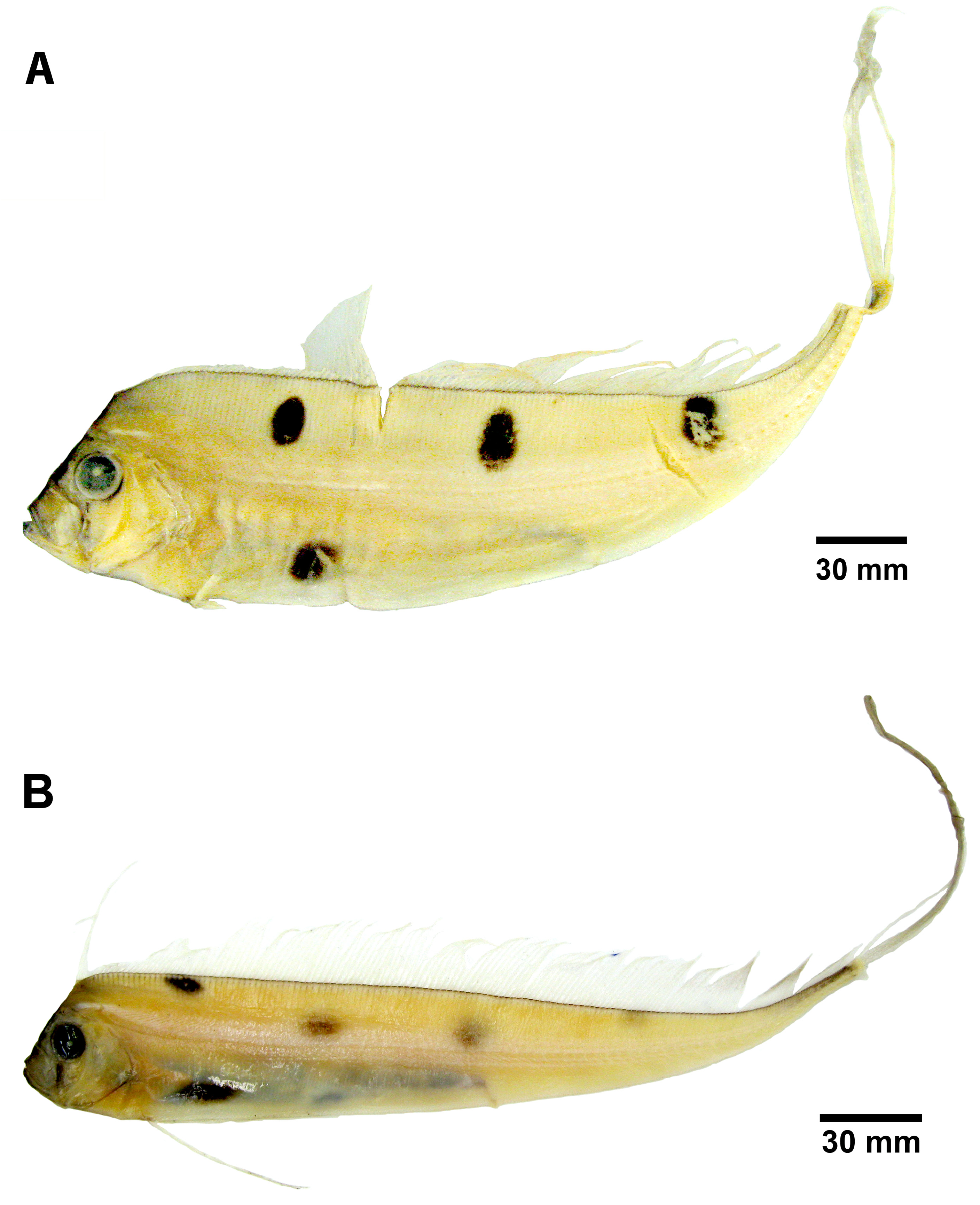
Color. In addition to reduction in fin-ray number and length and changes in body proportions, the greatest difference between juvenile and adult Trachipterus is in coloration pattern. As with adults, fins are red or crimson, the frontal profile from the dorsal fin origin to the tip of the lower jaw is black and the body is silver. However, juveniles most commonly possess several large, black spots on their lateral surfaces: there are typically 3–4 spots located dorsal to the lateral line and 1–2 located ventral to the lateral line ( Fig. 11 View FIGURE 11 ). Numerous species have been described based on variation in the number and location of the spotting pattern ( Emery 1879; Hamilton 1916) which appears to vary in regards to geography, ontogeny and, most likely, taxonomy.
Variation of the typical pattern includes 0–6 dorsal spots and 0–2 ventral spots. Establishing taxonomic identity based on juvenile spotting patterns is confounded by ontogeny associated with the color pattern. Spots are reduced in pigmentation, and eventually lost as standard length increases (see Ontogeny section below). Although a few exceptions have been noted, one of the most consistent geographical variations of the spotting pattern involves the location of the anterior-most dorsal spot. In specimens from the Atlantic Ocean and Mediterranean, the anteriormost dorsal spot is positioned below the dorsal midline. In specimens from the northern and southwestern Pacific, the first dorsal spot is located on the dorsal midline, in contact with the base of the dorsal fin. Specimens examined in this study collected from Tasmania and New Zealand exhibit the pattern described for Atlantic specimens ( Fig. 11A View FIGURE 11 ). However, specimens collected off eastern Australia in the Pacific Ocean follow the Pacific pattern ( Fig. 11B View FIGURE 11 ). Lateral asymmetry in spotting patterns, in which the spots are offset posteriorly on either the left or right side, also exists (BMNH 2010.3.23.21–26; NMNZ P.041259).
Juvenile to adult ontogenetic change. As with the other trachipterid genera, the transition from juvenile to adult does not appear to be correlated to size alone, and is hypothesized to occur over a longer time period, relative to Desmodema . Several morphological changes occur throughout development from juvenile to adult in Trachipterus , typically with several changes occurring simultaneously: 1) proportional elongation of body shape, which becomes more slender; 2) loss of the elongate dorsal-fin rays; 3) loss of the pelvic-fin rays; 4) reduction of the elongate dorsal caudal-fin rays; 5) reduction of ventral caudal-fin rays to rudiments; 6) reduction of anterior/lateral spinules on finrays; 7) increase in the prominence of dermal tubercles on the body and ventral midline; 8) loss of body spots.
Even without the opportunity to examine true adults, McCoy (1886:84) hypothesized that “ the young are deeper and shorter in proportion than the old. ” As standard length increases, head length, snout-vent length, and body depth at the pectoral-fin origin decrease, while tail length (= post-anus length; standard length- minus snout-vent length) increases ( Fig. 8 View FIGURE 8 ). Throughout ontogeny, individuals become proportionately more slender. Relative tail length also increases as a result of the lengthening of posterior vertebrae during ontogeny as successive posterior vertebrae are progressively longer. Meek (1890) first reported on an increase in the length of posterior vertebrae, relative to more anterior vertebrae. Walters & Fitch (1960) noted that those vertebrae in the mid-tail region are 2.5 to 4 times longer than those in the mid-trunk region.
As individuals progress through the juvenile stage and increase in length, the anterior-most (first 4–7) elongate dorsal-fin rays serially decrease in length, eventually reduced to a faintly detectable dorsal ridge that is the only evidence of the elongate fin-rays that once existed. This is also the case with the elongate rays of the dorsal caudal lobe. The rays of the ventral caudal lobe are reduced, first to spine-like rudiments ( Fig.6 View FIGURE 6 ) and eventually to a smooth lobe.
With the decrease in length of elongate fin rays, the lateral spinules on the dorsal-fin rays and the dorsal caudalfin rays, which are extremely numerous and prominent in early juveniles, also decrease to the point of nonexistence. Hamilton (1916:373) stated that “no radical change takes place on the surface of fin rays with increasing age” while he was attempting to align several ontogenetic stages of Trachipterus from New Zealand with the correct species name. Hamilton noted the potential use of this character as diagnostic and stated that “unless the adult forms…lose the granulations on the fin rays…there can be no identity… with a form like T. jacksonensis which has no spinules”. At that time, T. jacksonensis was the only nominal species from the southwestern Pacific based on an adult specimen, and therefore, was described as having no lateral spinules on the fin rays. However, this is not a diagnostic character but rather an ontogenetic one.
Although it is one of the first morphological transitions to begin, loss of the pelvic-fin rays such that only a pelvic slit exists is one of the last transitions to be completed. By 1250 mm SL, nearly all specimens examined had barely detectable pelvic-rays, reduced to the level of the ventral surface of the body. However, in at least one specimen of 1880 mm SL ( KPM 25081), rudiments of the first elongate pelvic fin-ray were visible. At lengths greater than 1880 mm SL, only the pelvic slit was visible and no trace of pelvic-fin rays was detected .
Loss of the characteristic spotting pattern of juvenile Trachipterus (typically 3–4 dorsal spots and 1 ventral spot) appears to be the most gradual and most protracted of ontogenetic transitions. The general trend is a decrease in total number of spots with an increase in SL ( Fig. 12 View FIGURE 12 ). Although there is some variation of the number and location of spots present in juvenile specimens, loss typically occurs posteriorly to anteriorly and then ventrally to dorsally. When only two spots are present on juveniles, they are the anteriormost dorsal spot and anteriormost ventral spot. No data exist regarding the size/stage and the appearance of spots, as they are not present in larval specimens. No spots were detected in specimens smaller than 51 mm SL.
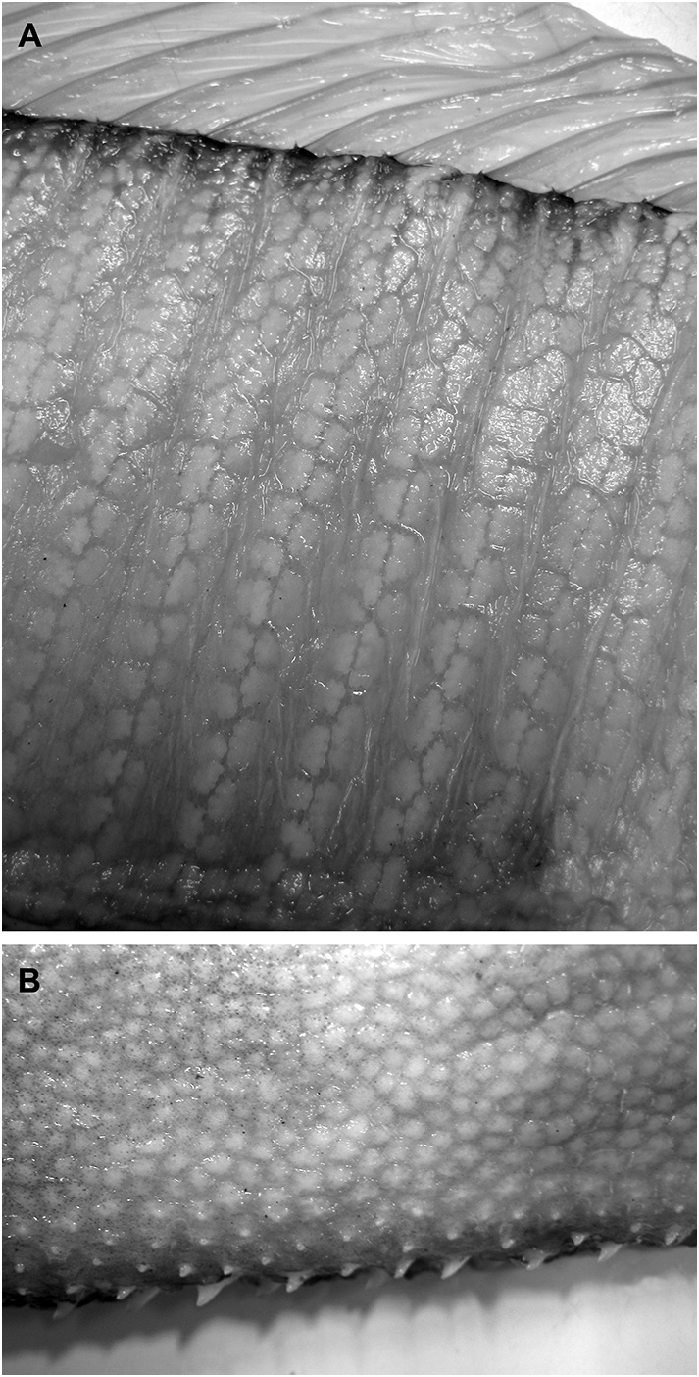
While most ontogenetically variable characters are marked with reduction, there is an increase in the prominence of tubercles across the body surface and along the ventral midline during ontogeny. Walters (1963) noted that these tubercles are cartilaginous in young Trachipterus but are bony in large adult specimens. Lateral tubercles were first detected at 215 mm SL (KPM 23327). As SL increases, the body tubercles become most distinct on the ventral midline, postanal region and along each side of the dorsal-fin pterygiophores ( Fig 13A View FIGURE 13 .). Walters (1963) hypothesized that the integumentary structure in trachipterids function as a drag-reduction mechanism by ensuring boundary-layer stability. The tubercles become greatly enlarged along the midventral line and project beyond the body surface ( Fig. 13B View FIGURE 13 .).
The adult stage is reached when the following characters are obtained: 1) adult proportions (as described above) are attained; 2) elongate dorsal-fin rays are non-existent; 3) pelvic fins are reduced to open slits with no detectable fin rays; 4) elongate dorsal caudal-fin rays are reduced in length; 5) ventral caudal-fin rays are reduced to rudimentary bases; 6) spinules are not present on the dorsal-fin rays and the dorsal caudal-fin-rays; 7) dermal tubercles are bony and project beyond the body on the ventral midline; and 8) no lateral spots are detected either dorsally or ventrally. Data on sexual maturity and reproductive behavior, which are greatly lacking from the literature, would contribute to better understanding of developmental transitions in Trachipterus .
Taxonomic history. The genus Trachipterus was established, without an included species, by Antoine Goüan in his 1770 work Histoire des Poissons. Goüan’s description for Trachipterus was likely based on a juvenile specimen(s). First, Goüan described a pelvic fin. In adult Trachipterus the pelvic-fin rays are reduced to bases in which no rudiments of the fin-rays are left externally. In contrast, pelvic fins of juveniles are long (“longer than the pectoral” as described by Goüan). The caudal-fin rays are described as elongate. This is likely a reference to the rays of the dorsal caudal lobe, which in juveniles are elongate and fan-like. Goüan’s original description noted the presence of “prickles” on the dorsal- and pelvic-fin rays and the caudal-fin rays as “rough”. These prickles likely refer to the spinules present on the fin rays. These spinules are reduced throughout ontogeny and are rarely detectable in adult fishes. Further, juvenile ribbonfishes are more abundant and are found in more nearshore habitats compared to adults, which are primarily offshore, deep-water fishes. Availability for collection due to this habitat difference likely contributed to the rarity of adult specimens in 18 th century zoological collections.
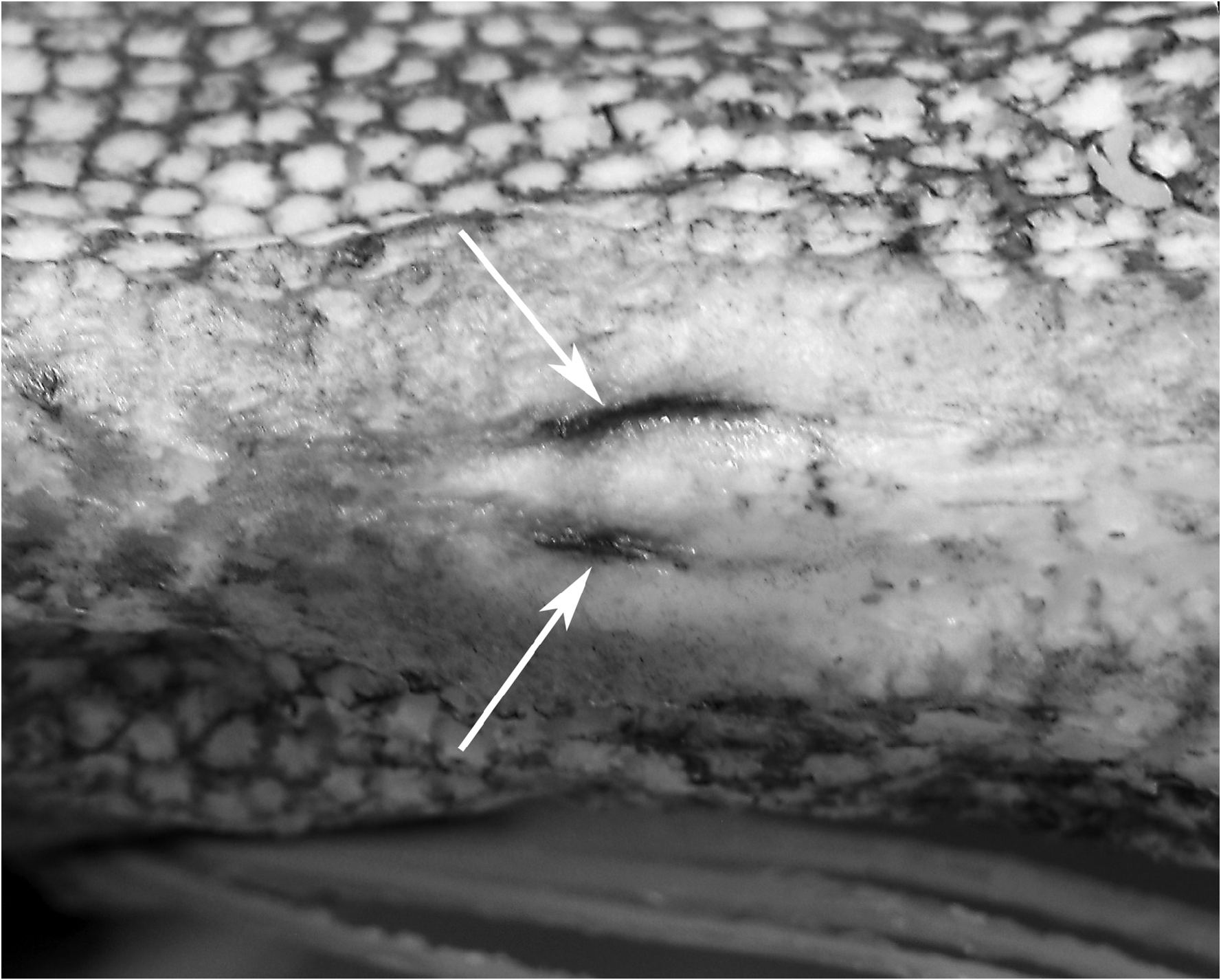
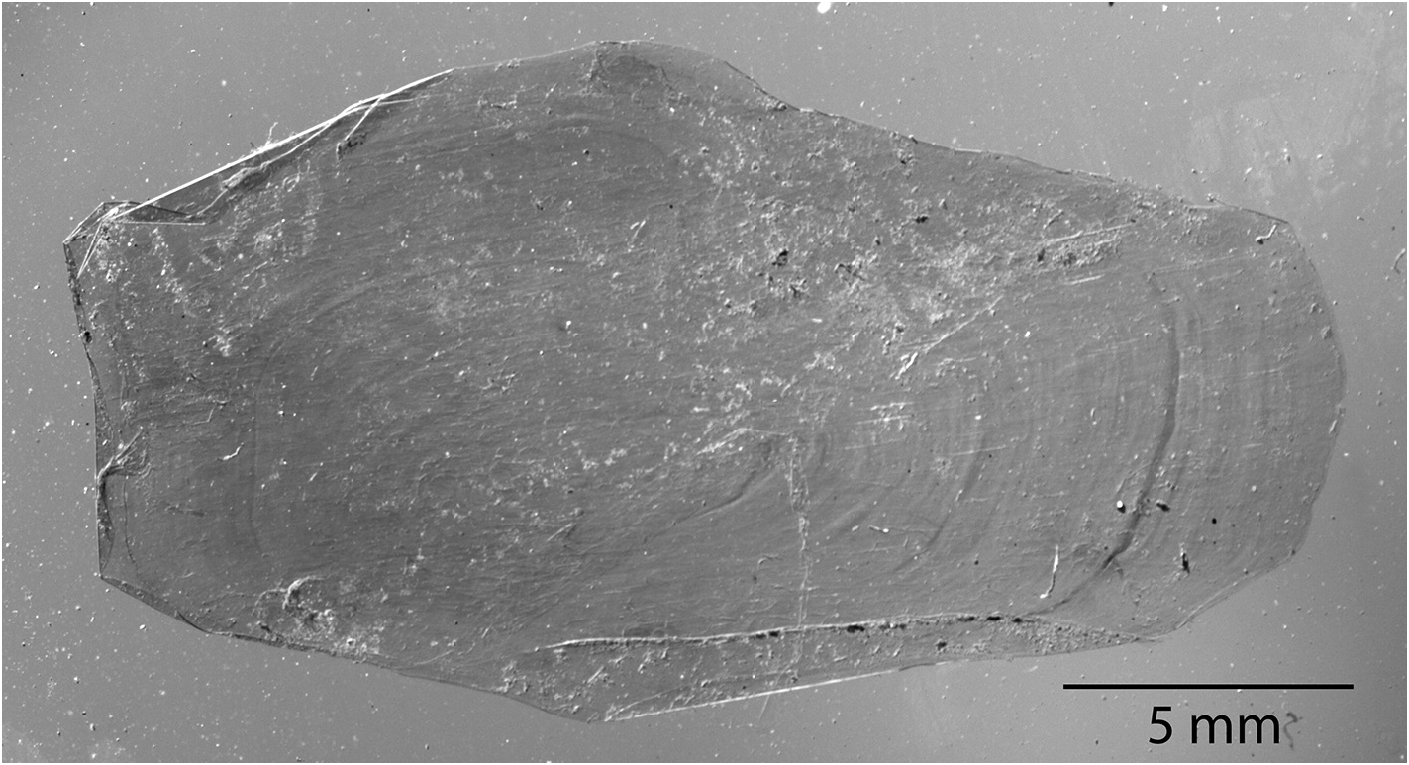
In Goüan’s original description, Trachipterus was defined as a genus in which the body is “ Squamae nullae ” (without scales). Many authors ( McCoy 1886; Walters & Fitch 1960; Palmer 1961; Fitch 1964; Scott 1983; Heemstra & Kannemeyer 1984) also note the absence of scales at all life stages as a diagnostic character for Trachipterus . This has in turn been used to distinguish Trachipterus from Zu, and according to some authors, Trachipterus from Desmodema ( Rosenblatt & Butler 1977) . However, Nishimura (1964) reported a Trachipterus specimen he identified as T. ishikawae as having a “body feebly covered with non-overlapping scales”. Nishimura (1964) noted that, after 10 months of preservation in formalin, there was no trace of squamation. Close examination of an adult specimen identified as T. jacksonensis that was caught on hook and line (therefore minimally damaged) from New Zealand (NMNZ P.41970; 1724 mm SL) revealed the presence of simple, fragile, non-overlapping cycloid scales on the lateral surface of the body (covered by the pectoral fins) and at the base of the dorsal-fin rays ( Fig. 14 View FIGURE 14 ). Upon further examination, scales were also found in a specimen identified as T. trachypterus (NMNZ P.16453; 1880 mm SL). Having been collected in a trawl, this specimen was severely damaged and scales were only detected on the lateral surface of the body covered by the pectoral fins. Scales have since been found in numerous specimens of Trachipterus spp. in sizes from 215 mm SL (KPM 23327) to 2472 mm SL (KPM 10429, a formalin-preserved specimen). Scales were originally detected during examination periods of at least 1 hour at which point scales would begin to lift at the edges, which is when they were observed. The scales are inconspicuous and potentially lost due to damage and preservation methods.
| NMNZ |
Museum of New Zealand Te Papa Tongarewa |
| CAS |
California Academy of Sciences |
| SU |
Stanford University |
| CSIRO |
Australian National Fish Collection |
| HUMZ |
Hokkaido University, Laboratory of Marine Zoology |
| MCZ |
Museum of Comparative Zoology |
| QVM |
Queen Victoria Museum |
| NMP |
National Museum (Prague) |
| KPM |
Kanagawa Prefectural Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.