Stevekenia, Percy, Diana M., 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4286.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:7D53A038-92BA-4F68-8326-8EB2D7C453BC |
|
DOI |
https://doi.org/10.5281/zenodo.6018161 |
|
persistent identifier |
https://treatment.plazi.org/id/03E15434-D74F-FFFD-FF5A-29B68CF2B20F |
|
treatment provided by |
Plazi |
|
scientific name |
Stevekenia |
| status |
gen. nov. |
Genus Stevekenia View in CoL View at ENA gen. nov.
Type species: Stevekenia nothocestri sp. nov., by present designation.
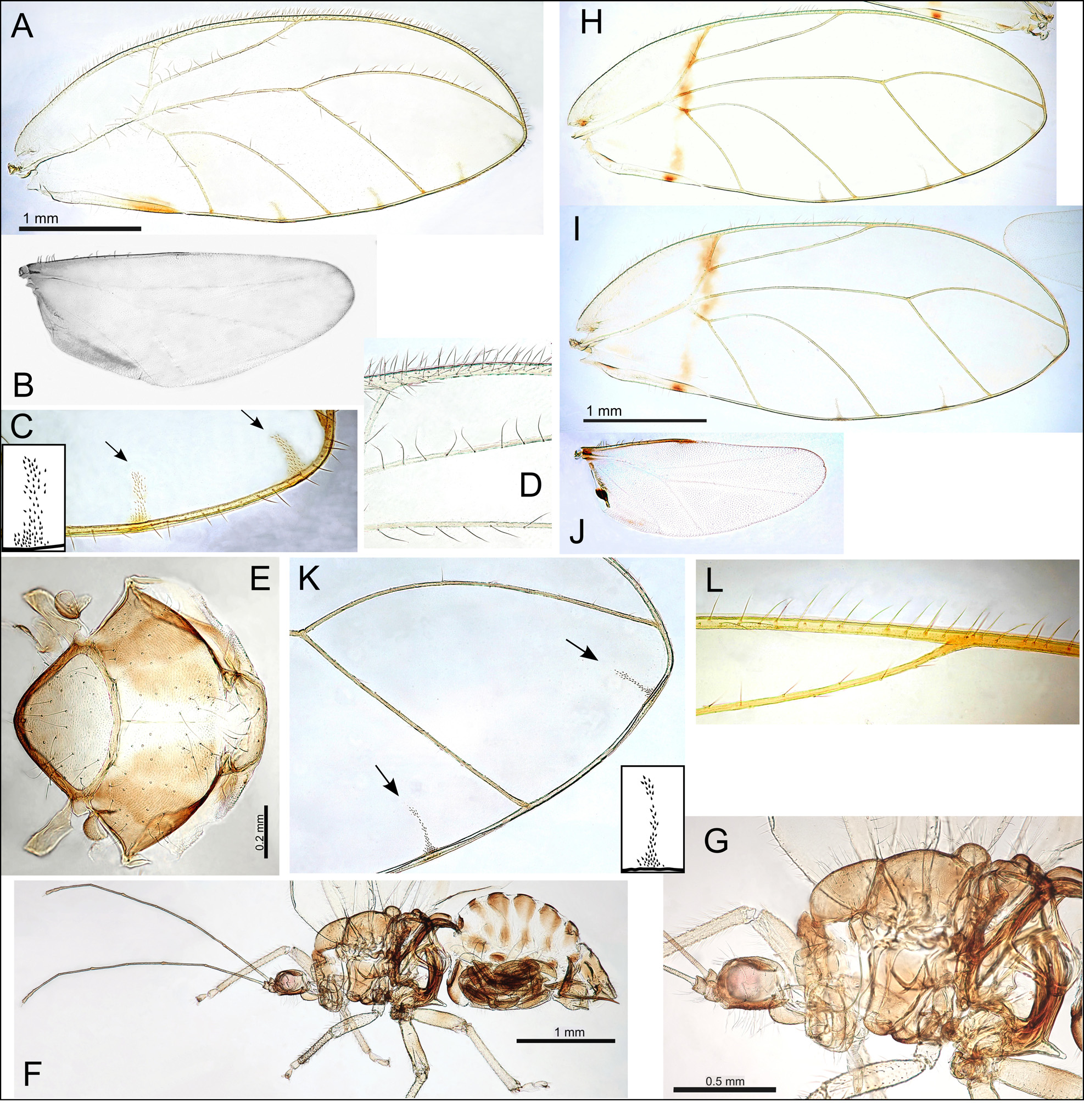
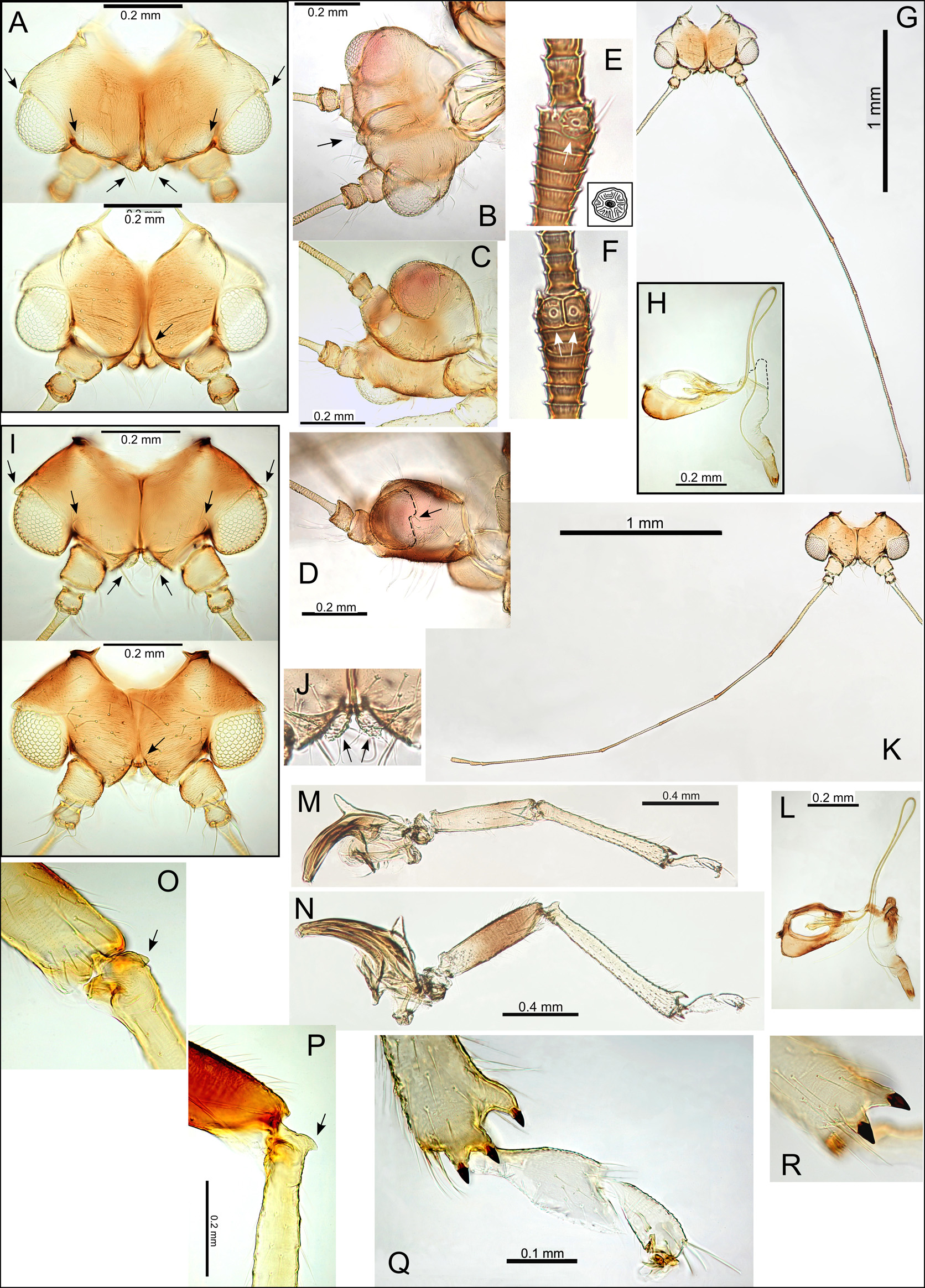
Adult colour and structure. General body colour light to mid-brown with yellow-green on abdomen. Fore wing broadest in the middle, membrane clear or slightly yellow and hyaline, lacking surface spinules, with or without distinct areas of pigmentation; veins with trifurcation of veins R, M and Cu1, brown, often with darker spots at marginal termination; long setae on ventral fore wing margin and interior veins; a single cluster of marginal radular spines present in cells cu1, m1, either centrally positioned or offset towards posterior of cells, and either one or two marginal clusters in cell m2; vein Rs short, cells cu1 and m2 large; fore wing apices bluntly acute ( Fig. 1A,C–D View FIGURE 1 ,H– I,L). Hind wing short ( Fig. 1B View FIGURE 1 ,J). Long setae present on head and thorax ( Fig. 1E View FIGURE 1 ). Head without genal processes; vertex extremely short (width ≥ 4x length), extending anteriorly into two small projections overhanging the medial ocellus ( Fig. 2A–B View FIGURE 2 ,I); due to the short vertex, the head appears somewhat dorso-ventrally flattened ( Fig. 2D View FIGURE 2 ); lateral ocelli on small tubercles; medial epicranial suture distinct; small extensions at posterior rim of the eyes form small lateral projections ( Fig. 2A View FIGURE 2 ,I). Antennae extremely long (length> 4x head width); antennal segments 10, with apical region of segments 3–8 often slightly darker; either a single or multiple rhinaria apically on segments 4, 6, and a single rhinarium apically on segments 8, 9; rhinaria either simple or surrounded by a small disk ( Fig. 2E,F View FIGURE 2 ); terminal segment with two setae of unequal length (stout and slender). Distal proboscis segment medium short, darker apically ( Fig. 2H View FIGURE 2 ,L). Thorax moderately arched ( Fig. 1F–G View FIGURE 1 ). Legs moderately short and robust, tibia longer than femur; hind leg with meracanthus well developed and straight; hind tibia with a single genual spine basally and 1+2 sclerotized apical spurs (single spur stalked and a pair of stalked spurs either conjoined at the base or not) and a comb of stout unsclerotized setae; proximal tarsus longer than distal tarsus ( Fig. 2 View FIGURE 2 M–R). Male terminalia with subgenital plate more or less rounded, or more angular; proctiger with moderate posterior lobe medially, length longer than paramere; paramere with broad base below an abrupt anterior angle leading to sickleshaped neck with apex directed anteriorly; distal aedeagus segment with large, bulbous apex; sperm pump large ( Fig. 3A View FIGURE 3 –G). Female terminalia with proctiger robust, dorsal surface more or less straight, longer than subgenital plate, vase-shaped anal ring composed of a mostly continuous double row of cells, and two raised pores (approximately same size as abdominal spiracles) flanking anal ring; subgenital plate ventral surface either more or less straight or with medial bulge ventrally, apex acute or bluntly acute; ovipositor without serrations ( Fig. 3 View FIGURE 3 H–N).
Egg. Pale to light brown, with a long pedicel and short tail, and a distinct plug-like structure at the base near the pedicel; egg surface (mostly dorsal surface) covered in irregularly clustered to linearly ordered cellular outgrowths ( Fig. 3 View FIGURE 3 O–Q).
Immature. Unknown.
Comment. There are no clear taxonomic affiliations with other genera in Triozidae . However, Stevekenia may be affiliated with Baeoalitriozus Li, 2011 based on forewing structure, particularly the large fore wing cell m2, and shortened hind wing; Baeoalitriozus occurs on both Asian and American continents, as well as in Africa ( Yang et al. 2013). Other possible affiliations are with Schedoneolithus Tuthill, 1959 , a monotypic genus from South America which has a Solanaceae host and head lacking developed genae ( Tuthill 1959), but the overall head shape is still not as atypical as in Stevekenia . Other than the host plant affiliation, the biology and immatures of Stevekenia are unknown. Ongoing phylogenomic work by this author places Stevekenia in a major clade of predominantly non-galling genera; and both Baeoalitriozus and Schedoneolithus are also in this same phylogenetic group, as is a predominantly holarctic genus, Bactericera Puton, 1876 , with several Solanaceae-feeding species, including one of the most serious pests of potato, Bactericera cockerelli ( Šulc, 1909) , see Discussion. Potentially significant similarities with Bactericera include the sickle-shaped paramere and eggs with a long pedicel, in addition some Bactericera taxa also lack developed genae ( Burckhardt & Lauterer 1997).
There are two single island endemic species described in Stevekenia , and the genus can be separated from other genera in the Hawaiian Islands by the combination of large size (> 4 mm), large fore wing cells m2 and cu1, extremely long antennae (> 4x head width), and the unusual, somewhat dorso-ventrally flattened, head shape without genal processes; as well as being the only taxon found on Solanaceae host plants in the archipelago. In addition, there are two notably unusual morphological features in Stevekenia , one is the two raised pores flanking the anal ring on the dorsal surface of the female proctiger, it is not clear whether these are simply pores or may function as glands; the other is the egg structure with branching cellular outgrowths on the surface of the eggs, and a plug-like structure at the base of the pedicel.
Etymology: Named for the combined efforts of two extraordinary field biologists, the entomologist Steve Montgomery and the botanist Ken Wood, without whose field knowledge and skills this genus would have remained undiscovered.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.