Hemiplasta mustea ( Bates, 1865 ), 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.5073.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:AA3269D1-CA2F-4528-BC9D-3A4C75D05BD9 |
|
DOI |
https://doi.org/10.5281/zenodo.5770805 |
|
persistent identifier |
https://treatment.plazi.org/id/03DB87EE-FF75-9DD1-FF40-5EFDFEAEF741 |
|
treatment provided by |
Plazi |
|
scientific name |
Hemiplasta mustea ( Bates, 1865 ) |
| status |
comb. nov. |
Hemiplasta mustea ( Bates, 1865) n. comb.
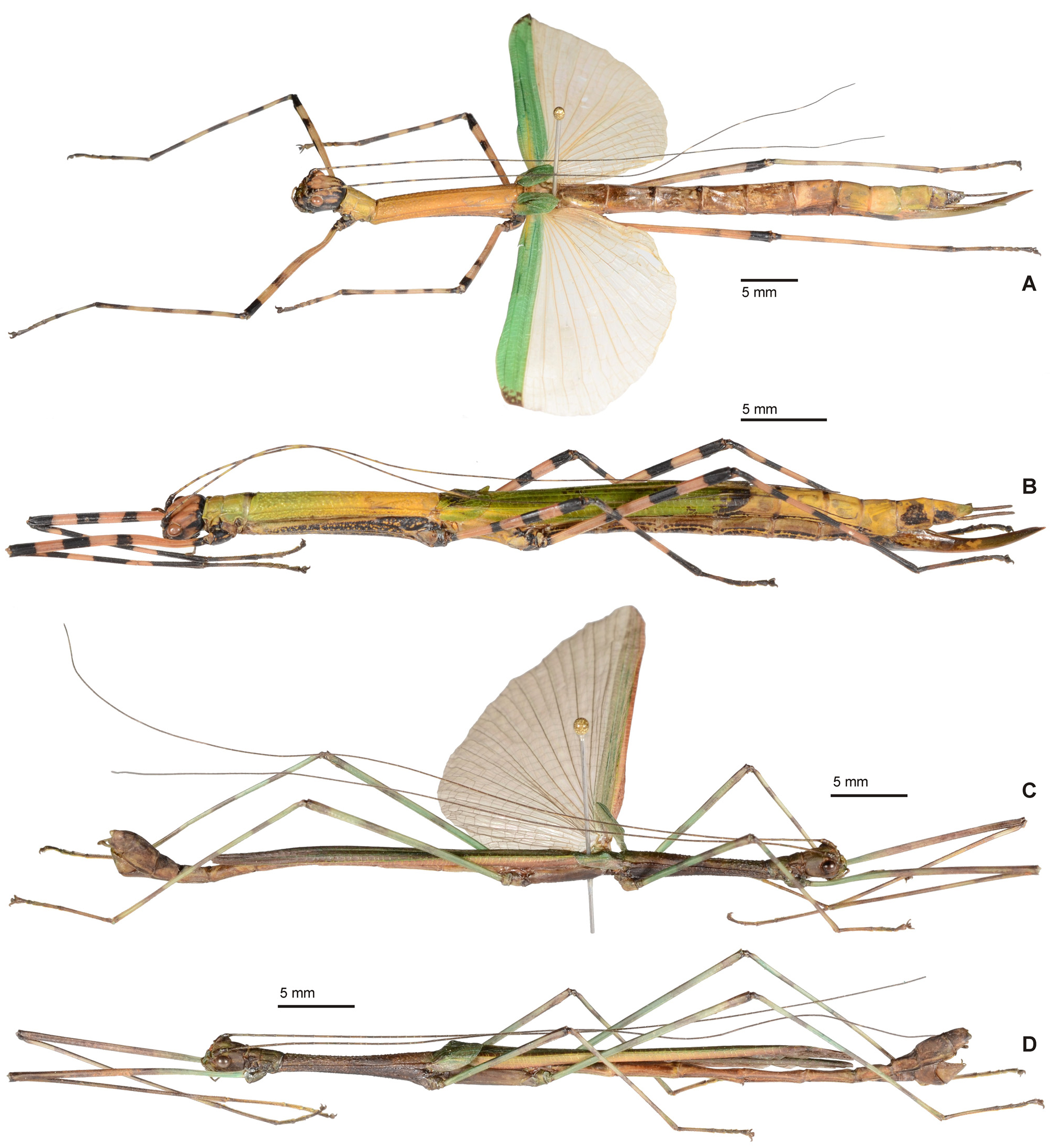
( Figs. 52–53 View FIGURE 52 View FIGURE 53 )
Necroscia mustea Bates, 1865: 355 , pl. 45: 8 (3). HT, ♂: Sula; Necroscia mustea Bates ♂; E. coll. (1830-73) W.W. Saunders, Purchased and pres ‘73 by Mrs. F.W. Hope [OXUM, No. 646].
Kirby, 1904: 377.
Marmessoidea mustea, Redtenbacher, 1908: 513 .
Bruner, 1915: 236.
Otte & Brock, 2005: 194.
Aruanoidea densegranulosa Redtenbacher, 1908: 521 . HT, ♂: Sula Besi, Doherty, ex coll. H. Fruhstorfer ; Insel östl. v. Celebes; Fruhstorfer vend. 25.X.1898.; 133 [ZMUH]. n. syn.
Bruner, 1915: 237.
Weidner, 1966: 228. (Catalogued)
Zompro, 2002: 184. (Catalogued)
Necroscia densegranulosa, Hennemann, 1998: 121 .
Otte & Brock, 2005: 211.
Sipyloidea (Hemiplasta) falcata Redtenbacher, 1908: 550 , pl. 27: 2 ( ♀). LT (by present designation), ♀: Sula Mangoli, Oct.– Novbr. Doherty, ex coll. H. Fruhstorfer [NHMW, No. 1089]; PLT, ♀: Sula Mangoli, Oct.–Novbr. Doherty, ex coll. H. Fruhstorfer ; Insel östl. v. Celebes; Fruhstorfer vend. 25.X.1898.; 153 [ZMUH]. n. syn.
Zompro, 2002: 186. (Catalogued)
Sipyloidea falcata, Bruner, 1915: 238 .
Weidner, 1966: 232.
Brock, 1998: 28.
Hemiplasta falcata, Hennemann, 1998: 121 , fig. 20 ( ♀).
Otte & Brock, 2005: 153.
Further material: 1 ♂: Indonesien, Banggai Arch., Peleng Island , II.2007 [coll. FH, No. 0297-33] ; 1 ♂: Indonesien, Banggai Arch., W-Peleng Island, Buko District , Tinanasu, XII.2012 [coll. FH, No. 0297-34] ; 1 ♀, 1 ♂: Indonesien, Banggai Arch., Peleng Id., Tinagkung Utara district , nr. Luksagu village, 60 m, 1°17’ S 123°25.4’E [coll. FH, No’s 0297-35 & 36] GoogleMaps ; 5 ♀♀, 5 ♂♂: Indonesien, Banggai Ids., W-Peleng Island, Buko District , btw. Tatendeng village and Eben village 400–550 m, IX.2011 [coll. FH, No’s 0297-37 to 46] ; 5 ♀♀, 5 ♂♂: Indonesien, Banggai Ids., W-Peleng Island, Buko District , btw. Tatendeng village and Eben village 400–550 m, IX.2011 [ IMQC] ; 10 ♀♀, 19 ♂♂, 1 ♀ (n5), 30 eggs: ex Zucht F. Hennemann 2008/09, Herkunft: Sulawesi , Peleng Island, leg. D. Dupont 2006 (PSG No. 285) [coll. FH, No’s 0297-2 to 31, E] ; 1 ♀: Indonesien, Prov. Maluka, Tanimbar Ids., Yamdena Id. VI.2007 [coll. FH, No. 0297-32—locality doubtful, see comment below] .
Differential diagnosis: Very similar to H. nigra ( Hennemann, 1998) n. comb. with which ♀♀ share the fairly stocky shape, slightly shortened alae and strongly elongated cerci ( Figs. 53A–C View FIGURE 53 ). Females however readily differ from those of H. nigra by their pretty and complex colouration, being mostly green with pink and black annulated legs and an orange, pink or red head that is in the posterior half variably furnished with black longitudinal streaks and markings ( Fig.53H View FIGURE 53 ); furthermore the ventral surface of the body is irregularly flecked with black. Morphologically they may be distinguished from H. nigra by the less acutely granulose and relatively longer mesothorax, which is about 5x longer than the prothorax (only 4x longer in nigra ), and differently shaped anal segment, which is somewhat broader with the posterolateral angles more triangular than in nigra ( Fig. 53B View FIGURE 53 ). Males differ from those of H. nigra by the dull blueish green general colour ( Fig. 53L View FIGURE 53 ), orange anterior margin of the alae, dull green and black annulated legs, slightly longer and less prominently granulated mesonotum ( Fig. 53G View FIGURE 53 ), more prominent and rather triangular median excavation of the posterior margin of the anal segment ( Fig. 53E View FIGURE 53 ) and broader, more obtusely rounded posterior margin of the poculum ( Fig. 53F View FIGURE 53 ).
Eggs ( Figs. 53J–K View FIGURE 53 ): The eggs have not yet been formally described, hence a description is provided here based on a good number of examples originating from the culture stock from Peleng.
Elongate, bullet-shaped, somewhat compressed laterally and oval in cross-section; about 2.8x longer than wide and 3.8x longer than hight. Anterior and ventral as well as lateral surfaces almost parallel-sided throughout most of the length, the polar area somewhat narrowed. Entire surface of capsule very minutely and fairly evenly granulose but with a few more distinct granules and rugulae anterior and posterior of the micropylar plate. Micropylar plate very elongate, slender and spear-shaped, widened sub-posteriorly and with the anterior 2/3 gradually narrowing towards and acutely pointed anterior end. The outer margin slightly swollen and raised and the ventral portion with a shallow longitudinal bulge; surface otherwise as that of capsule. Micropylar cup very smalla nd with a V-shaped ridge ventrally. Operculum elliptical, bent in lateral aspect and displaced towards the dorsal egg surface with the dorsal portion more narrowed the ventral portion; surface unevenly tuberculose with the outer rim smooth. Colour plain greyish mid brown, the outer margin of the micropylar plate and the V-shaped ridge of the micropylar cup dark brown. Measurements [mm]: Length 4.8–5.0, width 1.7–1.8, height 1.2–1.3, length of micropylar plate 2.5–2.6.
Comments: Bates (1865: 355) originally described N. mustea based on a unique ♂ holotype collected on the Sula Islands by Alfred Russel Wallace and now in the collection of OXUM. The species was subsequently transferred to Marmessoidea Brunner v. Wattenwyl, 1893 by Redtenbacher (1908) where it was retained since. Redtenbacher (1908: 550) originally described H. falcata based on two ♀♀ from Pulau Mangole, Sula Islands. The specimen in NHMW is here selected as the lectotype to guarantee stability of the name and justify the synonymy here introduced. Detailed examination of the ♂ holotype of Aruanoidea densegranulosa from the island of Sulabesi (= Sanana), the southeasternmost island of the Sula archipelago has shown this to be the opposite sex. Comparison of the type-specimen of A. densegranulosa with the holotype of N. mustea leaves no doubt they are the same species, hence both of Redtenbacher’s species become junior synonyms of the species originally described by Bates ( n. syn.). Like in two wild caught ♂♂ from Peleng in the author’s collection (coll. FH, No’s 0297-33 & 34), the holotype of mustea has the distinctive orange anterior margin of the costal region of the alae faded, which presumably was caused by provisional storage in ethanol. But also the captive reared specimens at hand show that this colour trait in particular tends to fade easily in dried specimens and is difficult to preserve properly. The distinct annulations of the legs seen in live insects appear also to fade notably when the specimens are dried and may almost disappear if liquids like ethanol are used for preservation.
A ♀ recorded from Sulawesi and attributed to H. falcata by Hennemann (1998: 114, fig 20) has proven to be the opposite sex of H. nigra (Henneman, 1998) n. comb. which is here transferred to Hemiplasta . Hence, H. densegranulosa has not yet been recorded from Sulawesi and appears to be restricted to the Banggai and Sula Islands. A ♀ in the author’s collection is said to be from Pulau Yamdena, the main island of the Tanimbar archipelago in the very southeast of Wallacea and almost 1000 km away from the Sula Islands. Since, this record is inexplicable in biogeographic aspecs and violates all known distributions of related biota within Wallacea, this record must be regarded as very doubtful and most certianly wrong. Possible “Tanimbar” was confused with Taliabu, the main island of the Sula archipelago.
Culture stock of H. densegranulosa was collected on the island of Peleng by D. Dupont ( France) in 2005 and the species is since then successfully reared in Europe. Various species of Hypericum (Fam. Hypericaceae ) are accepted as alternative food plants. Nymphs show colourations that are very different from that of adults, being various sahes of yellow and pale green flecked with black and white. Females use their long and sword-like subgenital plate to stick eggs into substrates like moss, lichens, bark or soil.
Distribution: Sula Islands: Pulau Mangole & Sanana “Sulabesi”; Banggai Islands: Pulau Peleng, Tinangkung Utara District, nr. Luksagu village; Buko District, Tinanasu; Buko District, btw. Tatendeng village & Eben village 400– 550 m.
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Tribe |
Stephanacridini |
|
Genus |
Hemiplasta mustea ( Bates, 1865 )
| Hennemann, Frank H. 2021 |
Necroscia densegranulosa
| Hennemann, F. H. 1998: 121 |
Hemiplasta falcata , Hennemann, 1998: 121
| Hennemann, F. H. 1998: 121 |
Sipyloidea falcata
| Bruner, L. 1915: 238 |
Necroscia mustea
| Bates, H. W. 1865: 355 |