Alitta acutifolia ( Ehlers, 1901 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3919.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:FCC72B2D-BA06-4B46-89B1-6D2D0388189D |
|
DOI |
https://doi.org/10.5281/zenodo.4631772 |
|
persistent identifier |
https://treatment.plazi.org/id/03D687AA-FF9F-EF17-FF73-FE687C2165EB |
|
treatment provided by |
Plazi |
|
scientific name |
Alitta acutifolia ( Ehlers, 1901 ) |
| status |
|
Alitta acutifolia ( Ehlers, 1901) View in CoL , reinst., n. comb.
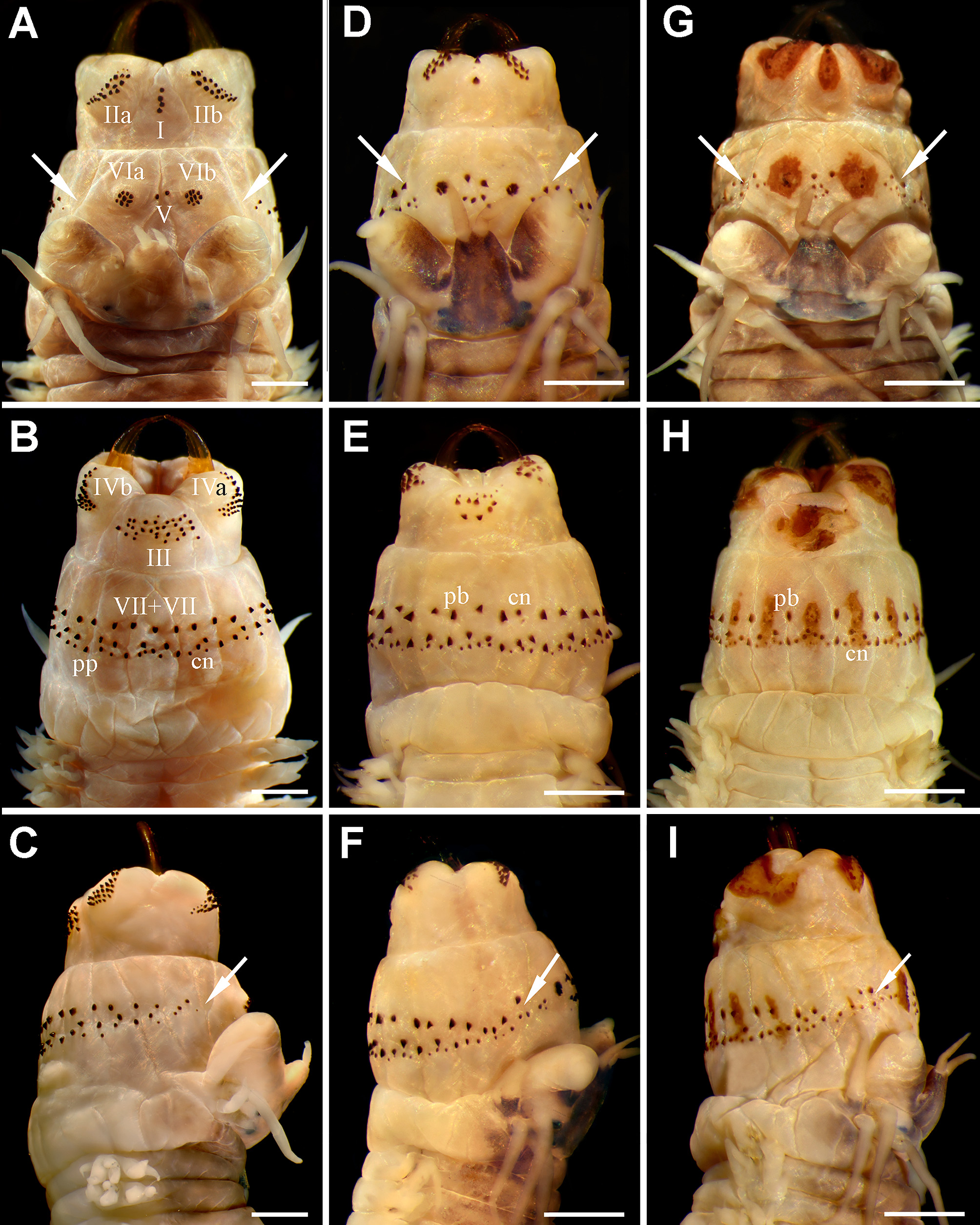
Figures 1 View FIGURE 1 D −I, 3
Nereis acutifolia Ehlers, 1901: 118 View in CoL −121, Pl. 13, Figs. 1 View FIGURE 1 −12; Hartwich 1993: 75 (partim).
Neanthes succinea Rioja 1947: 203 View in CoL ; 1962: 165; Hartmann-Schröder 1959: 142; de León-González & Solís-Weiss 2000: 556; Dean 2001: 51 −52, Figs. 30−33 (partim); Ferrando & Méndez 2010: 3 (non Leuckart, 1847).
Alitta succinea Tovar-Hernández et al. 2012: 8 View in CoL −9 (only diagnosis and figures); Villalobos-Guerrero 2012: 131−165, Figs. 1 View FIGURE 1 −5, 7 (only issues and figures related to samples from Sinaloa); Villalobos-Guerrero & Tovar-Hernández 2014: 65 −66 (only variations and habitat, non Leuckart, 1847).
Type material. Tropical Eastern Pacific, Guatemala. Three syntypes of Nereis acutifolia ( ZMB 6733), labeled “ Salvador, west coast of Guatemala ”, collected by Dr. Krefft ( Ehlers 1901:121), no more data. Measurements of syntypes are as follows: one complete atokous specimen with 78 chaetigers, TL= 22 mm, L15= 7.6 mm, W15= 1.4 mm; one incomplete atokous female fragmented into two pieces with 76 chaetigers, TL= 40 mm, L15= 14 mm, W15= 2.5 mm; and one complete epitokous male with 84 chaetigers, TL= 12 mm, L15= 5 mm, W15= 1.5 mm.
Additional material. Tropical Eastern Pacific, Costa Rica. ECOSUR-OH-P709-718, 10 specimens, Puntarenas, 9°58’27.35’’N, 84°49’52.71’’W, 22 Nov 2012, among barnacles, anemones and mytilids on concrete dock pilings, col. T. Villalobos. Gulf of California. ECOSUR-P2731, 12 specimens, Guaymas, Sonora, 27°54’02.5’’N, 110°51’15.8’’W, 11 Aug 2011, fouling on buoy, col. T. Villalobos & J. Aguilar. ECOSUR-P2732, 15 specimens, Topolobampo, Sinaloa, 25°35’33.4’’N, 109°04’04.9’’W, fouling on buoys, 0 9 Aug 2011, col. J. Aguilar & I. Ramírez. ECOSUR-P2733, 26 specimens, slaughterhouse, Urías estuary, Mazatlán, Sinaloa, 23º12’38.1’’N, 106º22’36’’W, metallic buoy, 0 3 Jun 2008, col. M. Tovar. ECOSUR-P2734, 13 specimens, off thermoelectric plant, Urías estuary, Mazatlán, Sinaloa, 23º11’04’’N, 106º21’25.6’’W, metallic buoy, 18 Aug 2008, col. M. Tovar & T. Villalobos. ECOSUR-P2735, 16 specimens, navigation channel, Urías estuary, Mazatlán, Sinaloa, 23º12’19.2’’N, 106º24’22.2’’W, metallic buoy, 18 Aug 2008, col. M. Tovar & T. Villalobos. ECOSUR- OH-P654, P656, 2 specimens, Urías estuary, Mazatlán, Sinaloa, 23°11’4.00’’N, 106°21’25.60’’W, fouling, 18 Aug 2008, col. M. Tovar & T. Villalobos. ECOSUR-OH-P655, P685, 2 specimens, Urías estuary, Mazatlán, Sinaloa, 23°10’47.57’’N, 106°25’15.31’’W, in oyster Crassostrea iridescens , 22 Mar 2011, col. T. Villalobos. Western Mexico. ECOSUR-OH-P658, P665, P666, 3 specimens, Bucerías, Nayarit, 20°45’40.15’’N, 105°21’45.15’’W, in oyster, 24 Mar 2013, col. J. Jarquín & P. Salazar. ECOSUR-OH-P638, P639, 2 specimens, Las Escolleras beach, Puerto Madero, Chiapas, 14°42’13.00’’N, 92°24’25.00’’W, in oyster Crassostrea prismatica , 11 Apr 2008, col. S. Salazar & L. Carrera.
Re-description based on atokous syntype (variations indicated in parentheses). Syntype with 78 chaetigers (n= 88, µ= 70.6±3.2, ran: 37−122), TL= 22 mm (n= 88, µ= 18.3±1.7, ran: 7−44), L15= 7.6 mm (n= 100, µ= 6.0±0.3, ran: 2.5−14.0), W15= 1.4 mm (n= 100, µ= 1.5±0.1, ran: 0.5−6.0).
Prostomium anteriorly complete ( Fig. 1 View FIGURE 1 D, G), 1.3 times longer than wide (µ= 1.2, ran: 1.0−1.4); mid-dorsal groove present. Palpophores suboval, slightly longer than wide (usually somewhat wider than long; µ= 1.1 times, ran: 0.8−1.5), slightly narrower than prostomium (µ= 0.9, ran: 0.7−1.3). Antennae close together, large, tapering, extending beyond palp tips ( Fig. 1 View FIGURE 1 D, G); extended posteriorly to mid-prostomium (0.5 times, ran: 0.3−0.8). Two pairs of eyes, short, similar sized, dark, rounded (anterior pair occasionally reniform). Upper peristomial cirri reaching chaetiger 3 (n= 80, µ= 4.1±0.2, ran: 2−6); lower peristomial cirri slightly exceeding palps (occasionally reaching tip). Upper tentacular cirri reaching chaetiger 5 (n= 80, µ= 6.6±0.4, ran: 4−10); lower tentacular cirri slightly exceeding half prostomium (frequently longer). Cirrophores of upper tentacular cirri 1.5 times longer than those from upper peristomial cirri (sometimes similar sized due to fixation). Apodous segment 1.5 times longer than length of chaetiger 1.
Pharynx with red amber jaws, 8 teeth (n= 71, µ= 8.5±0.2, ran: 7−10). Paragnaths conical, except some p-bars in areas VII −VIII. Paragnaths not emerging from a plate-like basement (occasionally a brownish orange soft platelike basement is present in both pharyngeal rings, except in area V; Fig. 1 View FIGURE 1 G −I); arranged longitudinally under conical paragnaths in VII −VIII, present in some ventral fleshy pads ( Fig. 1 View FIGURE 1 H, I). Maxillary ring: I= 5 (n= 71, µ= 2.6±0.2, ran: 2−5), in a longitudinal line ( Fig. 1 View FIGURE 1 D); IIa= 19 (n= 63, µ= 18.2±0.9, ran: 8−26), IIb= 18 (n= 63, µ= 18.0±0.9, ran: 10−25), two or three irregular curved rows with small-slender and large-coarse cones, the latter anteriorly located ( Fig. 1 View FIGURE 1 D); III= 16 (n= 63, µ= 13.5±1.0, ran: 6−23), three irregular rows in quadrangular group (sometimes four irregular rows forming a trapezoid; Fig. 1 View FIGURE 1 E); IVa= 23 (n= 61, µ= 21.2±1.3, ran: 12−32), IVb= 21 (n= 61, µ= 20.8±1.3, ran: 12−33), crescent-shaped, tapering anteriorly ( Fig. 1 View FIGURE 1 E). Oral ring: V= 8 (n= 70, µ= 5.0±0.5, ran: 0−11), without regular arrangement (usually two parallel rows, each one formed by two or three paragnaths; Fig. 1 View FIGURE 1 D, G); VIa= 9 (n= 68, µ= 8.5±0.4, ran: 4−14), VIb= 8 (n= 68, µ= 8.1±0.3, ran: 5−12), arranged in circular group with one concentric cone ( Fig. 1 View FIGURE 1 D; occasionally, no regular arrangement is distinguishable); VII −VIII= 87 (n= 71, µ= 75.3±4.0, ran: 44−115), two rows with cones and p-bars, anterior row continuous, with alternating combination of cones and bars, posterior row discontinuous, not interspersed, most posterior paragnaths smallest ( Fig. 1 View FIGURE 1 E, F, H, I). Bare space between VI and VII −VIII reduced, as wide as palpostyle or sometimes narrower, both regions almost joining ( Fig. 1 View FIGURE 1 F, I); paragnaths in VII −VIII present on a dorsal triangular fleshy pad next to VI ( Fig. 1 View FIGURE 1 D, F, G, I).
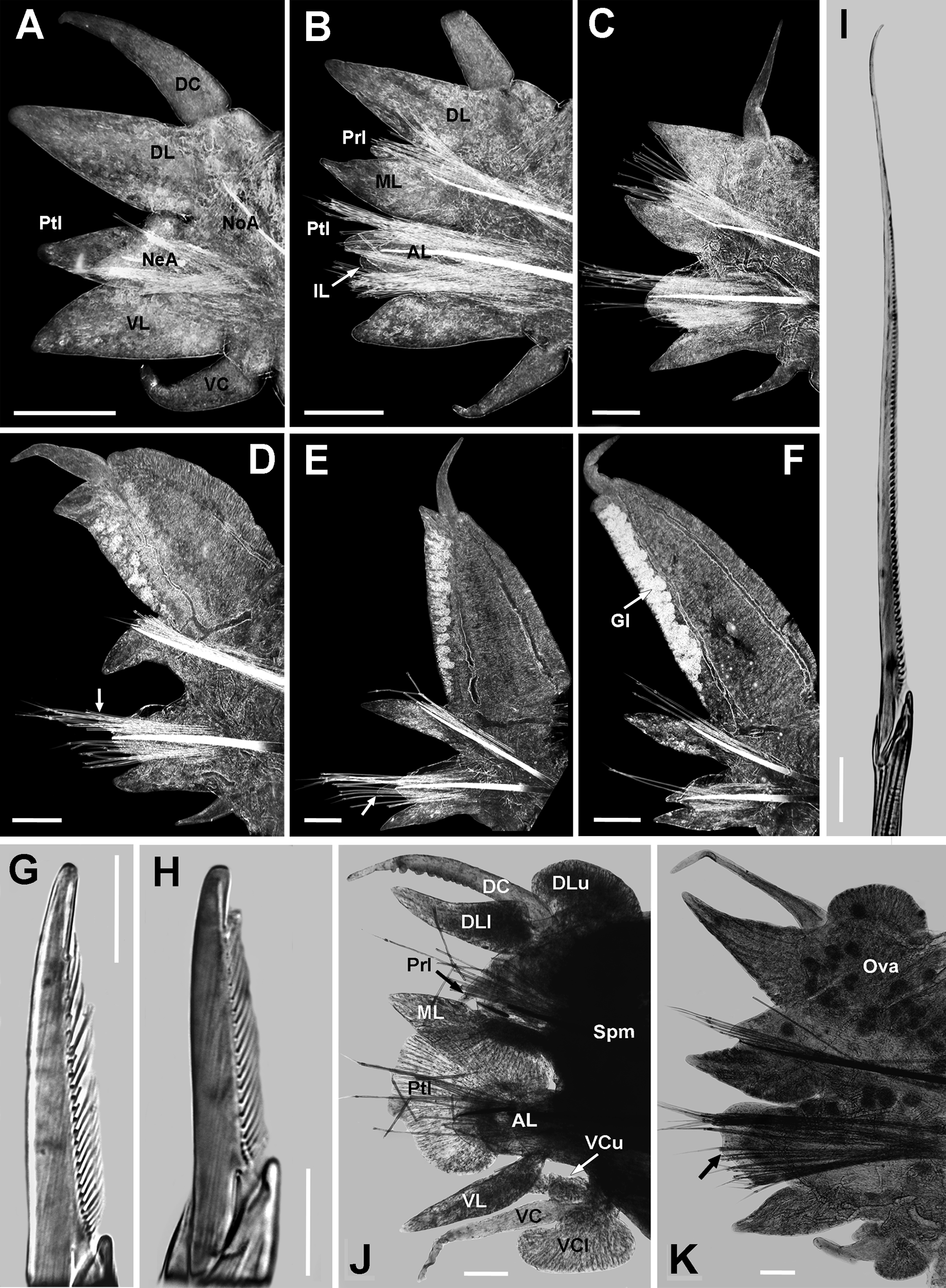
Biramous parapodia, except chaetigers 1 and 2, sub-biramous. Chaetigers 1 and 2 ( Fig. 3 View FIGURE 3 A) with dorsal cirrus slightly longer than dorsal ligule (occasionally as long), basally attached. Dorsal ligule conical, blunt tip, swollen, as long and thick as ventral ligule, 1.5 times wider than ligule. Neuracicular ligule small, nearly one-half length of ventral ligule. Neuropodial postchaetal lobe digitiform, medium sized (poorly developed in juveniles, long in large specimens), three-quarters length of ventral ligule (less than one-half in juveniles) and half as wide and thick. Neuropodial inferior lobe rounded, small, thick, one-half length of neuropodial postchaetal lobe. Ventral cirrus three-quarters length of ventral ligule.
Anterior chaetigers ( Fig. 3 View FIGURE 3 B, C) with dorsal cirrus distally displacing and elongating on dorsal ligule, 3 (2−3) times longer than dorsal ligule, mid-dorsally attached to it. Dorsal ligule from digitiform to conical shaped, thick, slightly longer and 1.5 times wider than lanceolate median ligule. Notopodial prechaetal lobe progressively widening and becoming slightly smaller; tapered (occasionally conical), blunt tip, 0.5 to 0.7 times length and 0.4 to 0.7 times width of median ligule (juveniles bearing notoacicular papilla before chaetiger 5). Notopodial prechaetal lobe in chaetigers 3 (5 in juvenile specimens) to 37−59, notoacicular papillae-like thereafter. Neuropodial postchaetal lobe becoming smaller, thinning and narrowing; conical (usually digitiform), 0.7 to 0.5 times length (shorter in juveniles) and thickness, and 0.8 to 0.4 times width of median ligule (poorly developed in juveniles). Neuropodial inferior lobe progressive and slightly thickening and widening; rounded, thick, half to as long as neuropodial postchaetal lobe; present in chaetigers 1 to 20 (19−24), neuracicular papillae-like thereafter. Neuracicular ligule small, 0.75 (0.5−0.75) times length of ventral ligule, nearly as wide as it. Ventral ligule conical, blunt tip, gradually tapering to lanceolate, nearly as long as median ligule, slightly wider and thicker than it. Ventral cirrus three-quarters length of ventral ligule.
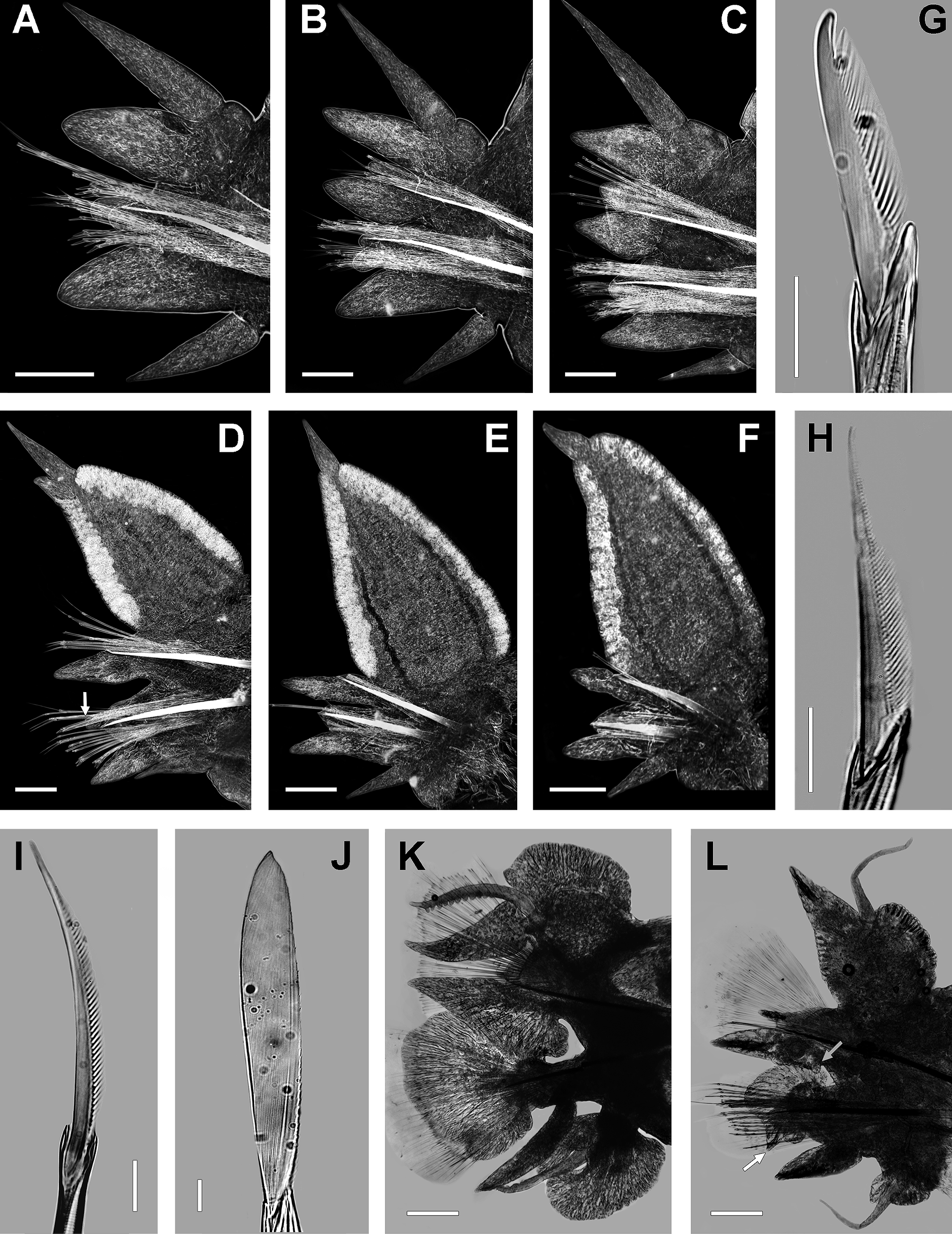
Mid-body chaetigers ( Fig. 3 View FIGURE 3 D) with dorsal cirri subterminal on dorsal ligule, becoming smaller and distally moving, 0.5 (0.4−0.5) times length of dorsal ligule; 2 (2−2.5) times longer than conical protruding tip of ligule. Dorsal ligule pennant-like, 1.5 times longer than wide; 3 (2−3) times longer and 3.5 (3.5−4) wider than lanceolate median ligule, reaching over three-quarters of width segment. Dorsal ligule expanding from about chaetiger 20, markedly elongate and wider on following chaetigers, with two glandular edges. Notopodial prechaetal lobe conical, 0.2 (0.2−0.3) times length and 0.5 (0.4−0.5) times width of median ligule. Neuropodial postchaetal lobe conical, 0.8 times length, 0.2 (0.2−0.3) width, and 0.4 (0.4−0.7) times thickness of median ligule; neuracicular papillae-like in last 22 (19−27) chaetigers. Neuracicular ligule one-half length of ventral ligule. Ventral ligule lanceolate, 0.8 times length of median ligule, slightly wider and slenderer than it. Ventral cirrus one-half length of ventral ligule.
Posterior chaetigers ( Fig. 3 View FIGURE 3 E, F) with dorsal cirri subterminal on dorsal ligule, displacing distally and becoming slightly smaller, 0.35 to 0.25 times length of dorsal ligule; terminal dorsal cirrus on last 18−22 chaetigers. Dorsal ligule pennant-like, 1.5 times longer than wide; 4 (3−4) times longer and 6 times wider than median ligule, clearly exceeding width of chaetiger; protruding tip vanished. Notopodial prechaetal lobe notoacicular papillae. Neuropodial postchaetal lobe neuracicular papillae. Neuracicular ligule 0.3 (0.3−0.5) times length of ventral ligule, slightly wider than it. Ventral ligule tapered, as long and wide as median ligule, slightly slenderer than it. Ventral cirrus as long as (usually one-half of) ventral ligule.
Notoaciculae on chaetigers 1 and 2 shorter, 0.3−0.5 times width of neuroaciculae, directed 10−30° respect to neuroaciculae. Similar sized and shaped as neuroaciculae in following chaetigers. Notochaeta homogomph spinigers throughout (only three specimens L15 <3 mm bearing irregularly long homogomph falcigers with terminal tendon on last 18 chaetigers). Spinigers of different sizes, long, medium and short blades ( Fig. 3 View FIGURE 3 I), except anterior chaetigers, without short blades (only present in juveniles); teeth long. Neurochaetae in upper fascicle with homogomph spinigers and heterogomph falcigers throughout (absent in juveniles). Falcigers long, somewhat wider than those from lower fascicle; teeth long, terminal tendon 1.5 times longer than other teeth. Spinigers of similar sizes from dorsal to ventral, long blades and teeth. Neurochaetae in lower fascicle with heterogomph spinigers and heterogomph falcigers throughout. Falcigers long with blades of different length, long on upper falcigers, shorter on lower ones; terminal tendon as long as other teeth ( Fig. 3 View FIGURE 3 G). Spinigers of different sizes with long teeth; long blades on upper spinigers, medium and short blades on lower ones ( Fig. 3 View FIGURE 3 H).
Pygidium with slender anal cirri, as long as last 11 chaetigers (n= 85, µ= 14.8±0.6, ran: 10−21); cirrophores well developed, coarse. Anus terminal, margin multidivided.
Live coloration. Prostomium and internal side of palps dark brown; dorsum of anterior segments reddish brown, gradually fading to pale brown on mid-posterior segments. Orange-yellowish dots randomly arranged over dorsum of parapodia and dorsolateral surface of segments. Pygidial cirri and anal circumference reddish brown. Images of anterior region of living specimens can be seen in Villalobos-Guerrero (2012) and Tovar-Hernández et al. (2012).
Preserved coloration. Dorsum of anterior segments reddish-brown, gradually blurring to pale brown on midposterior segments. Orange-yellowish dots faded. Dorsum of posterior segments bearing a central pale brown spot.
Syntype epitokous male (variations indicated in parentheses). Complete specimen with 84 chaetigers (n= 4, µ= 73.8±10.4, ran: 63−87), TL= 12 mm (n= 4, µ= 14.6±1.6, ran: 13.5−17), L15= 5 mm (n= 14, µ= 5.1±0.4, ran: 4−7), W15= 1.5 mm (n= 14, µ= 1.1±0.1, ran: 0.8−1.9). Body divided in three regions: pre-natatory, natatory and post-natatory. Eyes enlarged, oval, black, not overlapping (occasionally coalescent). Paragnaths well developed, coarse (sometimes rising from a plate-like basement, more conspicuous than in atokous). Pre-natatory region with 14 chaetigers. Dorsal and ventral cirri of first 7 and 5 parapodia modified, respectively. Parapodia 8 to 14 unmodified. Natatory region with 33 chaetigers (n= 14, µ= 29.7±1.6, ran: 24−33). Heteronereis morphology in mid-natatory parapodia as follows ( Fig. 3 View FIGURE 3 K): dorsal cirri as long as upper and lower lamella of dorsal ligule; 10 (9−11) bulbous papillae on lower edge, largest distally, terminal bare space equals width of three papillae. Upper lamella of dorsal ligule reniform, 3 times wider than long, terminal pointed tip, basal 75% attached to ligule. Lower lamella of dorsal ligule conical, slightly longer than median ligule, basal 40% attached to ligule. Notopodial prechaetal lobe short papilla. Median ligule lanceolate, as long as neuropodial postchaetal lobe, overlapping it. Neuropodial postchaetal lobe expanded, cordate, symmetrical, lower apex with blunt tip; lower edge wide, 2 (2−2.5) times width of neuracicular ligule. Neuracicular ligule 0.6 (0.5−0.7) times width of enlarged median ligule, with subdistal rounded upper edge. Ventral ligule fusiform, one-half width of neuracicular ligule, one-half length of neuropodial postchaetal lobe. Ventral cirri as long as ventral ligule; 9 (8−10) bulbous papillae on upper edge, similar sized, terminal bare space equals width of one papillae. Upper lamella of ventral cirri slightly longer than lower lamella, 0.4 times width of it. Lower lamella reniform, 2 times wider than long, blunt tip, basal 60% attached to cirrus. All atokous chaetae replaced by dense tuft of sesquigomph natatory chaeta with flat paddle-like blade; notochaetae finely serrated on lower edge, neurochaetae finely serrated on upper edge ( Fig. 3 View FIGURE 3 J). Post-natatory region with 13−34 chaetigers, one-half width of pre-natatory chaetigers. Pygidium unmetamorphosed, anus without papillae or pygidial rosette. Anal cirri shorter than in atokous.
Non-type epitokes females. All incomplete specimens, L15= 5−11 mm (n= 14, µ= 8.1±0.8), W15= 1−3 mm (n= 14, µ= 2.2±0.3). Body divided in three regions: pre-natatory, natatory and post-natatory. Eyes enlarged, oval, black, not overlapping. Paragnaths well developed, coarse, sometimes arising from a plate-like basement, more conspicuous than in atokous. Pre-natatory region with 17 chaetigers. Dorsal cirri of first 5 parapodia (6 in a single specimen) modified; ventral cirri of first 2−3 parapodia poorly modified. Parapodia 6 (7 in a single specimen) to 17 unmodified. Natatory region with 15−24 chaetigers (n= 14, µ= 18.4±2.1). Heteronereis morphology in midnatatory parapodia as follows ( Fig. 3 View FIGURE 3 L): dorsal cirri smooth, 1.5 times longer than upper and lower lamellae of dorsal ligule. Dorsal ligule transformed, but not greatly as in males; upper lamella slightly reniform, 3−4 times wider than long, blunt tip, basis fully attached. Lower lamella of dorsal ligule conical, slightly longer than median ligule, basis fully attached. Notopodial prechaetal lobe tapered. Median ligule lanceolate, lower edge flat, basal with a rounded papilla; extending beyond neuropodial postchaetal lobe, slightly overlapping it. Neuropodial postchaetal lobe expanded, but not greatly as in males, cordate, asymmetrical, lower apex with an enlarged conical tip. Neuracicular ligule 2 times wider than ventral ligule, with subdistal rounded upper edge. Ventral ligule lanceolate, as long as neuracicular ligule. Ventral cirri smooth, slightly longer than ventral ligule. Upper lamella of ventral cirri slightly longer than lower lamella, 0.4 times width of it. Lower lamella reniform, 1.5 times wider than long, blunt tip, basis fully attached. Atokous chaetae replaced by dense tuft of sesquigomph natatory chaetae, with flat paddle-like blade; notochaetae finely serrated on lower edge, neurochaetae finely serrated on upper edge. Postnatatory region with 17−66 chaetigers, one-half width of pre-natatory chaetigers. Pygidium unmetamorphosed. Anal cirri nearly as long as in atokous. Oocytes size: 100−122 µm (n= 100, µ= 114.9±0.9).
Habitat. Estuaries and harbors, 0−11 m depth. Soft bottoms, epibiont in native oyster Crassostrea iridescens (Hanley) and mangrove roots ( Rhizophora mangle L.) ( Hartmann-Schröder 1959; Villalobos-Guerrero 2012). Fouling on artificial substrates as buoys, ship hulls and dock pilings. Salinity: 32−36 ups, temperature: 20−32°C, dissolved oxygen: 3.0− 6.7 mg /L ( Villalobos-Guerrero & Tovar-Hernández 2014).
Type locality. Probably Acajutla, El Salvador. Ehlers (1901:121) cited the following distribution: “West-Küste von Guatemala, Salvador; San Jose de Guatemala; Punta Arenas, Costarica ”; nevertheless, we consider as syntypes only those specimens collected in “ Salvador, west coast of Guatemala ” because they were used in Ehlers’ description of the species. The precise type locality is uncertain; there is no place in the west coast of Guatemala named Salvador. “ Salvador ” could correspond to the country, El Salvador, which during 1824−1839 was part of the Federal Republic of Central America, with Guatemala as capital. If this is correct, it is possible that Acajutla, one of the most important and historical coastal places of El Salvador, could be the type locality.
Distribution. Central American Pacific, Mexican Tropical Pacific, Gulf of California.
Remarks. Ehlers (1901) described the species based on several atokous and two epitokous males. According to Hartwich (1993:75), there are five syntypes in the Zoologisches Museum, Berlin (ZMB 67333 [sic]), but we only found three (ZMB 6733): one epitokous male, one fragmented atokous female and one immature specimen. Hartwich likely confused the fragmented female and an Oxydromus sp. ( Hesionidae ) specimen. The epitokous male is clearly that described and drawn by Ehlers according to length (12 mm) and number of chaetigers (85); and the incomplete atokous female is likely that referred by Ehlers as the longest complete atokous specimen (43 mm, 84 chetigers), but broken and without pygidium (40 mm, 76 chaetigers). The remaining immature specimen was not mentioned and the second epitokous male (18 mm, 84 chetigers) was not found. The original material from San Jose ( Guatemala) and Puntarenas ( Costa Rica) is lost, although we were able to study additional new specimens from the latter locality. The specimens fully agree with the descriptions and illustrations performed by Ehlers.
The paragnath number in N. (Neanthes) acutifolia from Paita, Peru ( Gravier 1909) is similar to the original description, but other diagnostic characters were not provided. The record deserves confirmation. Monro (1933) followed Fauvel (1923a) placing N. acutifolia in synonymy with N. (N.) succinea , thus introducing the name into the Eastern Pacific ( Panama). Since then, N. acutifolia has been trated by subsequent authors as invalid. Hartman (1959) confirmed the synonymy of N. acutifolia under N. succinea based only on published accounts. After reviewing the type and topotype material, we recognize N. acutifolia as a valid species and transfer it to Alitta for the aforementioned reasons (see remarks of Alitta ).
The morphological differences between the species include 29 characters of immature and reproductive specimens. The species differ in the following 18 atokous diagnostic features: 1) Paragnaths arrangement in A. acutifolia n. comb. is II= 17−19, III= 12−14, IV= 20−22, V= 5−6 (seldom 0), and VII −VIII= 71−79; instead, A. succinea bears II= 19−22, III= 32−37, IV= 22−25, V= 1−3 (seldom 4), and VII −VIII= 50−55. 2) Bare space between areas VI and VII −VIII in A. acutifolia n. comb. is much reduced (as wide as palpostyle; Fig. 1 View FIGURE 1 D, G), whereas in A. succinea is wider (as wide as palpophore; Fig. 1 View FIGURE 1 A). 3) Alitta acutifolia n. comb. has alternating cones and p-bars in area VII −VIII ( Fig. 1 View FIGURE 1 E, H), while A. succinea has conical and pyramidal paragnaths ( Fig. 1 View FIGURE 1 B). 4) Alitta acutifolia n. comb. has dorsal cirrus at least 2−3 times longer than dorsal ligule on anterior chaetigers ( Fig. 3 View FIGURE 3 C), while the dorsal cirrus in A. succinea is as long as ligule on anterior chaetigers ( Fig. 2 View FIGURE 2 C). 5) Dorsal cirrus in A. acutifolia n. comb. is terminal on dorsal ligule on last 18−22 chaetigers, whereas in A. succinea is terminal on last 7−14 chaetigers. 6) A. acutifolia n. comb. has expanded dorsal ligule with glandular structures in both edges ( Fig. 3 View FIGURE 3 D −F); which are only present in lower edge in A. succinea ( Fig. 2 View FIGURE 2 D −F). 7) Dorsal ligule on posterior chaetigers clearly exceeds width of chaetiger in A. acutifolia n. comb.; however, dorsal ligule in A. succinea is shorter, does not exceed width. 8) Neuropodial postchaetal lobe in A. acutifolia n. comb. is absent in last 19−27 chaetigers ( Fig. 3 View FIGURE 3 E, F), while in A. succinea it is absent in the last 6−12 chaetigers. 9) Blades of some notopodial homogomph spinigers are short in A. acutifolia n. comb., in contrast to long ones in A. succinea . 10) Lower peristomial cirri in A. acutifolia n. comb. are longer (extending beyond palp tips) than in A. succinea (as long as palps or shorter). 11) Lower tentacular cirri in A. acutifolia n. comb. are longer (markedly exceeding half of prostomium) than in A. succinea (reaching half of prostomium or shorter). 12) Antennae in A. acutifolia n. comb. are thick, large, as long or slightly extending beyond palp tips ( Fig. 1 View FIGURE 1 D, G), extended posteriorly to reach one-half of prostomium; whereas in A. succinea antennae are slender, small, barely reaching half of palps ( Fig. 1 View FIGURE 1 A) and they are one-third length of prostomium. 13) Prostomium is longer than wide in A. acutifolia n. comb., while in A. succinea is as long as wide. 14) Eyes in A. acutifolia n. comb. are smaller than in A. succinea . 15) Jaws are red amber in A. acutifolia n. comb., while in A. succinea they are yellow amber. 16) Alitta acutifolia n. comb. occasionally has paragnaths atop a soft plate-like basement, which is absent in A. succinea . 17) Alitta acutifolia n. comb. is one-third length of and has fewer chaetigers than A. succinea . 18) Mid-dorsal surface of posterior chaetigers in A. acutifolia n. comb. has a brownish spot, while A. succinea lacks this pattern.
Alitta acutifolia n. comb. differs from A. succinea in 11 epitokous diagnostic features on mid-natatory chaetiger. Females: 19) First 2−3 ventral cirri in A. acutifolia n. comb. are modified, whereas the first 4 are modified in A. succinea . 20) Alitta acutifolia n. comb. exhibits 11−23 natatory chaetigers, against 30−33 chaetigers in A. succinea . 21) Alitta acutifolia n. comb. shows a short papilla in lower basis of median ligule ( Fig. 3 View FIGURE 3 L, grey arrow), while A. succinea lacks this feature. 22) Alitta acutifolia n. comb. displays an enlarged conical lower apex on postchaetal neuropodial lobe ( Fig. 3 View FIGURE 3 L, white arrow); while A. succinea has a short lower apex ( Fig. 2 View FIGURE 2 K, black arrow). 23) Oocytes in A. acutifolia n. comb. are bigger (113−115 µm) than those from A. succinea (100−103 µm). Males: 24) Alitta acutifolia n. comb. has 23−34 natatory chaetigers, and A. succinea has 37 natatory chaetigers. 25) Alitta acutifolia n. comb. has bulbous papillae on upper edge of ventral cirrus ( Fig. 3 View FIGURE 3 K); meanwhile papillae are on lower edge of ventral cirrus in A. succinea ( Fig. 2 View FIGURE 2 J). 26) Alitta acutifolia n. comb. has upper lamella of dorsal ligule with terminal pointed tip ( Fig. 3 View FIGURE 3 K), while A. succinea has upper lamella with blunt tip ( Fig. 2 View FIGURE 2 J). 27) Ventral cirrus as long as ventral ligule in A. acutifolia n. comb. ( Fig. 3 View FIGURE 3 K); however, in A. succinea ventral cirrus is longer than ventral ligule ( Fig. 2 View FIGURE 2 J). 28) Dorsal cirrus as long as upper and lower lamellae of dorsal ligule in A. acutifolia n. comb. ( Fig. 3 View FIGURE 3 K), whereas in A. succinea the dorsal cirrus extends beyond lower lamella and this is 2 times longer than upper lamella of dorsal ligule ( Fig. 2 View FIGURE 2 J). 29) Ventral ligule one-half length of neuropodial postchaetal lobe in A. acutifolia n. comb. ( Fig. 3 View FIGURE 3 K), while in A. succinea the ventral ligule is three-quarters length of lobe ( Fig. 2 View FIGURE 2 K).
The unusual melted paragnaths ( Bakken et al. 2009), or hard plate-like basement of paragnaths ( Glasby et al. 2011), are known in two species: Neanthes pachychaeta (Fauvel, 1918) and N. thysanota ( Ehlers, 1920) ( Glasby et al. 2011) . The structure is well developed under conical paragnaths of maxillary and (occasionally) oral rings in atokous and epitokous forms; it has been regarded as a taxonomically important character ( Glasby et al. 2011). Alitta acutifolia n. comb. displays a similar feature, more than being a melted paragnath plate, is a soft plate-like basement. Paragnaths may be present or absent because cones tend to fall as basements become rough and stiff. Soft plate-like basement occur randomly in the species, atokous and epitokous males and females may have or lack this conspicuous feature (which is absent in syntypes and is absent or present in specimens from Western Mexico and Costa Rica); therefore, basal plates are not regarded as a sexual difference. All features, except this basement of paragnaths in some specimens, agree with the original description and type material.
Morphological differences between A. succinea and A. acutifolia n. comb. are further supported by molecular data, since they have a genetic divergence of 27.5% ( Fig. 4 View FIGURE 4 ). Intraspecific genetic divergence in specimens of A. acutifolia n. comb. from Mexican Pacific and Central American Pacific was 0.6% (n= 5). Genetic divergence between both species is higher than those considered distinguishing species of polychaetes (8.4% to 21%) ( Jones et al. 2008; Vrijenhoek et al. 2009; Carrera-Parra & Salazar-Vallejo 2011). Furthermore, A. acutifolia n. comb. has a high genetic divergence with respect to A. virens from Atlantic Ocean (26.6%) and Alitta sp. from Canadian Pacific (26.9%).
Based on our morphological and molecular data, we regard A. acutifolia n. comb. as a valid species with a distribution in the Tropical Eastern Pacific. Moreover, we reject the supposed introduction of A. succinea , as an alien invasive species, in this region. Many records of A. succinea (some as Neanthes or Nereis ) from the Mexican and Central American Pacific are likely A. acutifolia n. comb. (e.g., Monro 1933; Rioja 1947, 1962; de León- González & Solís-Weiss 2000; Villalobos-Guerrero 2012; Villalobos-Guerrero & Tovar-Hernández 2014). The description of N. succinea from various depths and substrates from the Pacific of Costa Rica ( Dean 2001) is similar to A. acutifolia n. comb.; however, the size of Dean’s specimen (length: 259 mm, width: 19 mm, 91 chaetigers) exceeds by far the size range of the species (length: 7−44 mm, width: 0.5−6 mm, 122 chaetigers). Re-examination of the largest specimen reported by Dean (2001) is required to assess some diagnostic features and corroborate the identification.
| ZMB |
Museum für Naturkunde Berlin (Zoological Collections) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Nereidinae |
|
Genus |
Alitta acutifolia ( Ehlers, 1901 )
| Villalobos-Guerrero, Tulio F. & Carrera-Parra, Luis F. 2015 |
Alitta succinea Tovar-Hernández et al. 2012 : 8
| Villalobos-Guerrero 2014: 65 |
| Tovar-Hernandez 2012: 8 |
Neanthes succinea
| Ferrando 2010: 3 |
| Dean 2001: 51 |
| Leon-Gonzalez 2000: 556 |
| Hartmann-Schroder 1959: 142 |
| Rioja 1947: 203 |
Nereis acutifolia
| Hartwich 1993: 75 |
| Ehlers 1901: 118 |