Litoria gracilis, Richards & Donnellan & Oliver, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5263.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:9EF23FE9-DDD8-46D4-A275-09A35243BF30 |
|
DOI |
https://doi.org/10.5281/zenodo.7814486 |
|
persistent identifier |
https://treatment.plazi.org/id/1E175A08-DD09-44DA-9BF4-D044F0914691 |
|
taxon LSID |
lsid:zoobank.org:act:1E175A08-DD09-44DA-9BF4-D044F0914691 |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria gracilis |
| status |
sp. nov. |
Litoria gracilis , sp. nov.
Slender Spotted Treefrog
Figs 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7
https://zoobank.org/ urn:lsid:zoobank.org:act:
Holotype. SAMA R71697 ( SJR2297 ) Adult male with vocal slits and nuptial pads, small stream adjacent to road ascending Iagifu Ridge nr Moro, Southern Highlands Province, Papua New Guinea ( 6.3681°S, 143.2232°E; 940 m a.s.l.) by S.J. Richards on 21 October 2001. GoogleMaps
Paratypes (n = 12). PNGNM ( FN SJR8854 ) , SAMA R71673–4 ( FN SJR2066–2067 ), 71677 ( FN SJR4930 ), 71678 ( FN SJR8855 ), 71698 ( FN SJR2347 ) same details as holotype except R71673–4 collected on 15 May 2002 , R71677 collected on 30 September 1999 , PNGNM ( FN8854 ) and R71678 on 13 November 2004 and R71698 on 22 October 2001 ; R71699 ( FN SJR2361 ), adult male, Summit of Iagifu Ridge , Southern Highlands Province, Papua New Guinea ( 6.4446°S, 143.2193°E; 1360 m a.s.l.) by S.J. Richards on 22 October 2001 GoogleMaps ; R71679–71682 ( FN SJR10390 , 10423–10424 , 10438 ) , PNGNM ( FN SJR)10422, adult males except R71681 adult female, Juha South , Western Province, Papua New Guinea ( 5.9018°S, 142.4360°E; 950 m a.s.l.) by S.J. Richards 18–20 February 2008 GoogleMaps .
Diagnosis. Litoria gracilis sp. nov. is distinguished from all other Litoria by the following unique combination of characters: size small (SVL of males 25.1–28.2 mm, of one female 28.6 mm); dorsum in life brown with green spots; vomerine teeth not visible; finger webbing extensive; prominent dermal fold along outer edge of foot; pigmentation on nictitating membrane restricted to narrow band at dorsal margin; advertisement call a short bleating sound lasting 0.13– 0.28 s containing 2–5 distinctly pulsed notes produced at a rate of 7.51–12.00 notes/s with dominant frequency at 3800–4000 Hz; and is genetically diagnosable from L. daraiensis sp. nov. at 46 sites and from L. nigropunctata at 95 sites in the 787 base pair alignment of mitochondrial ND4 gene and flanking tRNA ( Table 1 View TABLE 1 ).
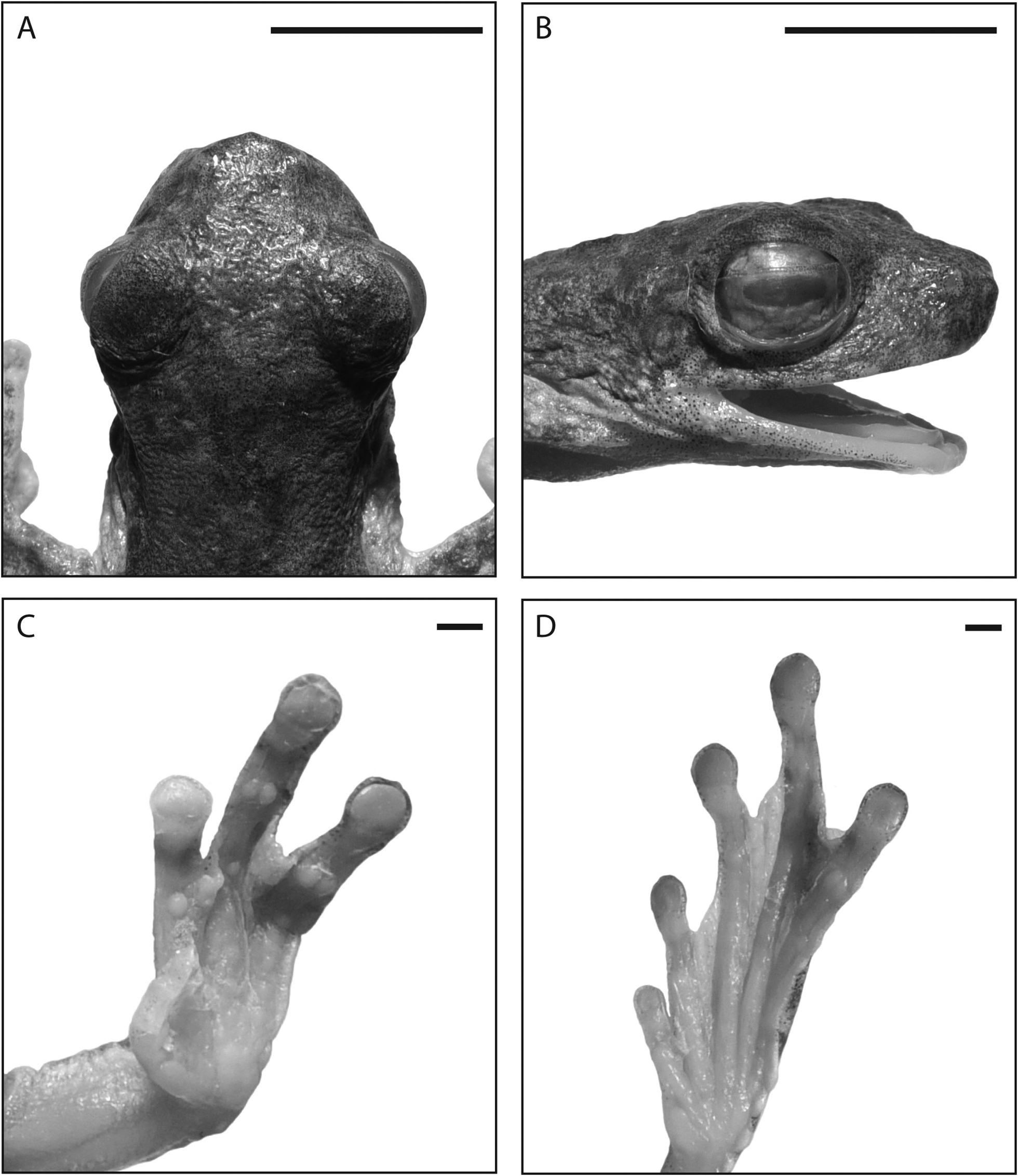
Description of holotype: An adult male with nuptial pads and vocal slits. Morphometric data are summarised in Table 3 View TABLE 3 . Body very slender. Limbs moderately long (TL/SVL 0.52). Head moderately narrow (HW/SVL 0.26), slightly wider than long (HL/SVL 0.25, HL/HW 0.96). Vomerine teeth not visible but detectable by scraping with forceps. Tongue oval with shallow posterior notch. Vocal slits long, located laterally in floor of mouth. Snout broadly rounded with angular tip in dorsal view ( Fig. 5A View FIGURE 5 ), steeply sloping (near truncate) in lateral view ( Fig. 5B View FIGURE 5 ); canthus rostralis short, broadly rounded, slightly curved; loreal region steeply sloping, slightly concave; lips slightly flared; nostrils much closer to tip of snout than eyes, oriented laterally; internarial distance greater than distance from external naris to eye (EN/IN 0.82, IN/SVL 0.10, EN/SVL 0.08); eyes moderately small (EYE/SVL 0.11) but prominent, protruding in dorsal and lateral views; pupil horizontal, pigmentation on nictitating membrane restricted to narrow band along dorsal edge. Tympanum small (TYM/SVL 0.04), slightly more than one third diameter of eye (TYM/EYE = 0.39), moderately well-defined except dorsal edge obscured by short supratympanic ridge.
Skin of dorsum and dorsal surfaces of limbs finely shagreened, throat finely ridged, abdomen coarsely granular. Ventral surfaces of limbs mostly smooth except numerous low but conspicuous white tubercles extending from vent distally to approximately halfway along postero-ventral margins of thighs; a dense cluster of prominent white tubercles surround vent and clump of small, low, pale tubercles on each heel; low, interrupted, white dermal fold extends along outer edge of tarsus and foot, extending from heel nearly to disc of Toe 5 but reduced to low ridge distally; row of small pale tubercles along distal-most outer edge of forearm grading into low dermal ridge along outer edge of hand, reaching nearly to base of disc on Finger 4.
Fingers moderately short with expanded terminal discs (3FD/SVL 0.05) with distinct marginal grooves; distalmost subarticular tubercles on fingers 3 and 4 strongly bilobed, those on fingers 1 and 2 unilobed ( Fig. 5C View FIGURE 5 ); relative lengths of fingers: 3>4>2>1. Fingers extensively webbed, on Finger 4 webbing extending slightly beyond distal edge of subarticular tubercle at base of penultimate phalanx (distal subarticular tubercle), on outer edge of Finger 3 to about distal subarticular tubercle before continuing as narrow flange to base of disc, on outside of Finger 3 to slightly beyond halfway between distal subarticular tubercle and disc, and between fingers 1 and 2 reduced to basal fringe ( Fig. 5C View FIGURE 5 ). Inner metacarpal tubercle low, elongate, outer metacarpal tubercle broad, flat. Nuptial pad pale brown, narrow, extending 1.5 mm along outer edge of Finger 1. Toes with relative lengths 4>3=5>2>1. Webbing on inside of Toe 5 and outside of Toe 3 extending nearly to base of discs, on both sides of Toe 4 to distal edge of distalmost subarticular tubercle, on outside of Toe 2 to halfway between distal subarticular tubercle and disc, and on Toe 1 to distal subarticular tubercle. Inner metatarsal tubercle well developed, oval; no outer metatarsal tubercle. Distalmost subarticular tubercles on toes 4 and 5 bilobed, on Toe 3 partially bilobed, and on toes 1 and 2 unilobed ( Fig. 5D View FIGURE 5 ). Tips of fingers and toes expanded into terminal discs, those of fingers slightly broader than those of toes (3FD/4TD 1.08), all with distinct marginal grooves.
Colour in life. Pale creamy brown dorsally and laterally, overlain with numerous small green spots; small patches of brown pigmentation concentrated on dorsal surfaces of tibiae and on outer edges of tarsi; ventrolateral surfaces behind axillae with fine peppering of brown pigment; posterior surface of upper arm largely yellow; dermal folds and large tubercles surrounding vent white. Ventrally white except ventral surfaces of forelegs and upper hindlegs; fine peppering of brown pigment along lateral edges of jaw; bright yellow in groin.
Colour in preservative. Dorsal surfaces faded pale blue with darker blue and brown patches, ventral surfaces cream, bright yellow surfaces in life faded to very pale yellow.
Variation. Morphometric variation is limited ( Table 3 View TABLE 3 ). The largest specimen is a female, but sexual dimorphism is slight (SVL of 12 males 25.1–28.2 mm, of one female 28.6 mm). All specimens are very slender ( Fig. 6A–C View FIGURE 6 ), and in life exhibited a yellow patch on the posterior surfaces of the upper arm, yellow in the hidden surfaces of the legs and on the posterior of the abdomen, and green spots on the dorsum. However, the size and number of green spots was highly variable, as was the density and extent of brown pigmentation and its aggregation into discrete patches of different sizes; some specimens had limited dark-brown pigmentation (e.g., Figs 6A–B View FIGURE 6 ) while others exhibited numerous discrete blotches of dark brown on the dorsum (e.g., Fig. 6C View FIGURE 6 ) and dorsal surfaces of the limbs.
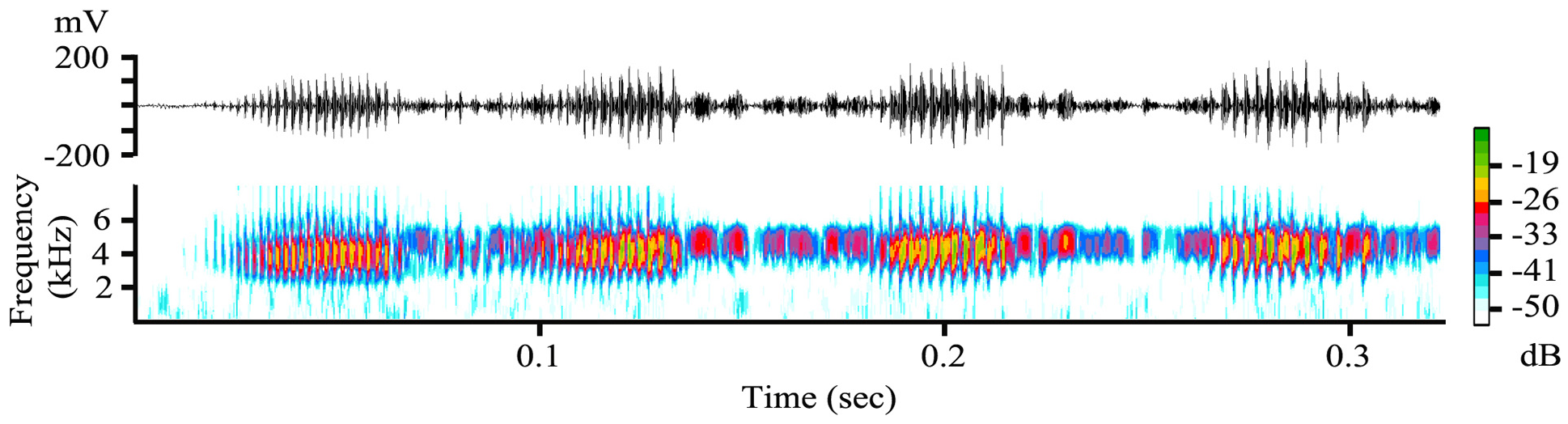
Advertisement call. We analysed nine calls produced by the holotype at an air temperature of 21.0°C and one call produced by SAMA R71680 ( Fig. 7 View FIGURE 7 ) at an air temperature of 21.2° C. The call of L. gracilis sp. nov. is a series of 2–4 (occasionally 5) finely pulsed notes. Calls were 0.13– 0.28 s long (mean = 0.24, SD = 0.06, n = 10) and those of the holotype were produced at 9.0– 44.3 s intervals over a period of 211 s (mean inter-call interval = 25.85 s, SD = 13.66, call rate = 0.04 calls/s). Of the 10 calls analysed in detail two (20%) contained two notes, seven (70%) contained three notes and one (10%) contained four notes ( Fig. 7 View FIGURE 7 ). Eighteen additional calls from an unvouchered male that were not analysed in more detail due to their poorer quality contained 2–5 notes (two notes = 22% of calls, three notes = 39%, four notes = 33%, 5 notes = 6% (a single call)). Notes within calls were produced at a rate of 7.51–12.00 notes/s (mean = 9.52, SD = 1.38, n = 10). Note length for all 10 calls combined was 0.033 –0.057 s (mean = 0.040, SD = 0.005, n = 29) and internote interval within calls was 0.011 –0.105 s (mean = 0.062, SD = 0.025, n = 19). In all calls the last internote interval was longer than preceding intervals ( Fig. 7 View FIGURE 7 ). Notes contained 11–23 pulses (mean = 15.09, SD = 3.06, n = 21) produced at a rate of 263–458 pulses/s (mean = 359.3, SD = 50.9, n = 21). Dominant frequency was 3800–4000 Hz.
Comparisons. In its small size (male SVL 25.1–28.2 mm, a female 28.6 mm), slender body, green and brown dorsal colour, extensively webbed fingers, and lacking a rostral spike, Litoria gracilis sp. nov. most closely resembles the following nine species: L. aplini , L. daraiensis sp. nov., L. iris , L. majikthise , L. nigropunctata , L. richardsi , L. singadanae , L. umarensis and L. verae . Litoria gracilis sp. nov. is also morphologically similar to the three new species described below and is compared with them in their respective species accounts.
Litoria gracilis sp. nov. differs from L. aplini by its smaller body size (adult males 25.1–28.2 mm vs. males 31.9–35.1 mm SVL), less prominent dermal fold along outer edges of limbs, hidden surfaces of limbs predominantly yellow (vs. predominantly blue with dark brown mottling), and advertisement call comprising 2–5 finely pulsed notes (vs. advertisement call a short buzz normally followed by 1–7 clicks); from L. iris by having hidden surfaces of limbs predominantly yellow (vs. posterior of thighs blue, red, or yellow, frequently blotched with white or purple), violet spots in axilla and groin absent (vs. present) and advertisement call a series of 2–5 finely pulsed notes (vs. advertisement call a series of up to 10 notes of variable length, with long notes preceding or following short notes); from L. majikthise by its smaller size (adult males 25.1–28.2 mm vs. 30–35 mm SVL), having posterior surfaces of thighs yellow (vs. uniform red), by lacking a pearl-white post-ocular bar (vs. present), lacking violet patches on posteroventral surfaces of abdomen (vs. present) and advertisement call a series of 2–5 finely pulsed notes (vs. advertisement call a single long (0.29– 0.36 s) or short ( 0.026 –0.080) note); from L. nigropunctata by its smaller, though slightly overlapping, size (male SVL 25.1–28.2 vs. 27–32 mm; Menzies 1972, 2006; Günther 2004), dermal folds along outer margins of tarsi distinctly crenulated (vs. dermal folds forming low dermal ridge), advertisement call a series of 2–5 finely pulsed notes (vs. advertisement call comprising an irregular succession of clicks and buzzes; Menzies 1972), and deep genetic divergence (( dA between the taxa of 0.15; see below); from L. richardsi and L. singadanae in having a smaller (TYM/EYE 0.32–0.42 vs. 0.65–0.81), pigmented (vs. substantially transparent) tympanum, and further from L. richardsi in lacking (vs. having) irregular black lines on dorsum and extensive black markings ventrolaterally, and from L. singadanae in lacking (vs. having) extensive area of orange on posteroventral surfaces in life; from L. umarensis in having posterior surfaces of thighs yellow (vs. brown), and dorsum brown with green spots (vs. normally uniform green); and from L. verae in its smaller size ( L. verae males 33–35 mm SVL), lacking (vs. having) small brown spots aligned transversely on dorsum, and having yellow (vs. orange) on posteroventral surfaces in life. The new species is most similar to L. daraiensis sp. nov., described above. It can be distinguished from that species by its slightly larger size (male SVL 25.1–28.2 vs. 23.9 mm), different advertisement call (a series of 2–5 finely pulsed notes vs. 10–11 distinctly pulsed chattering notes), and moderately deep genetic divergence ( dA between the taxa of 0.08; see below).
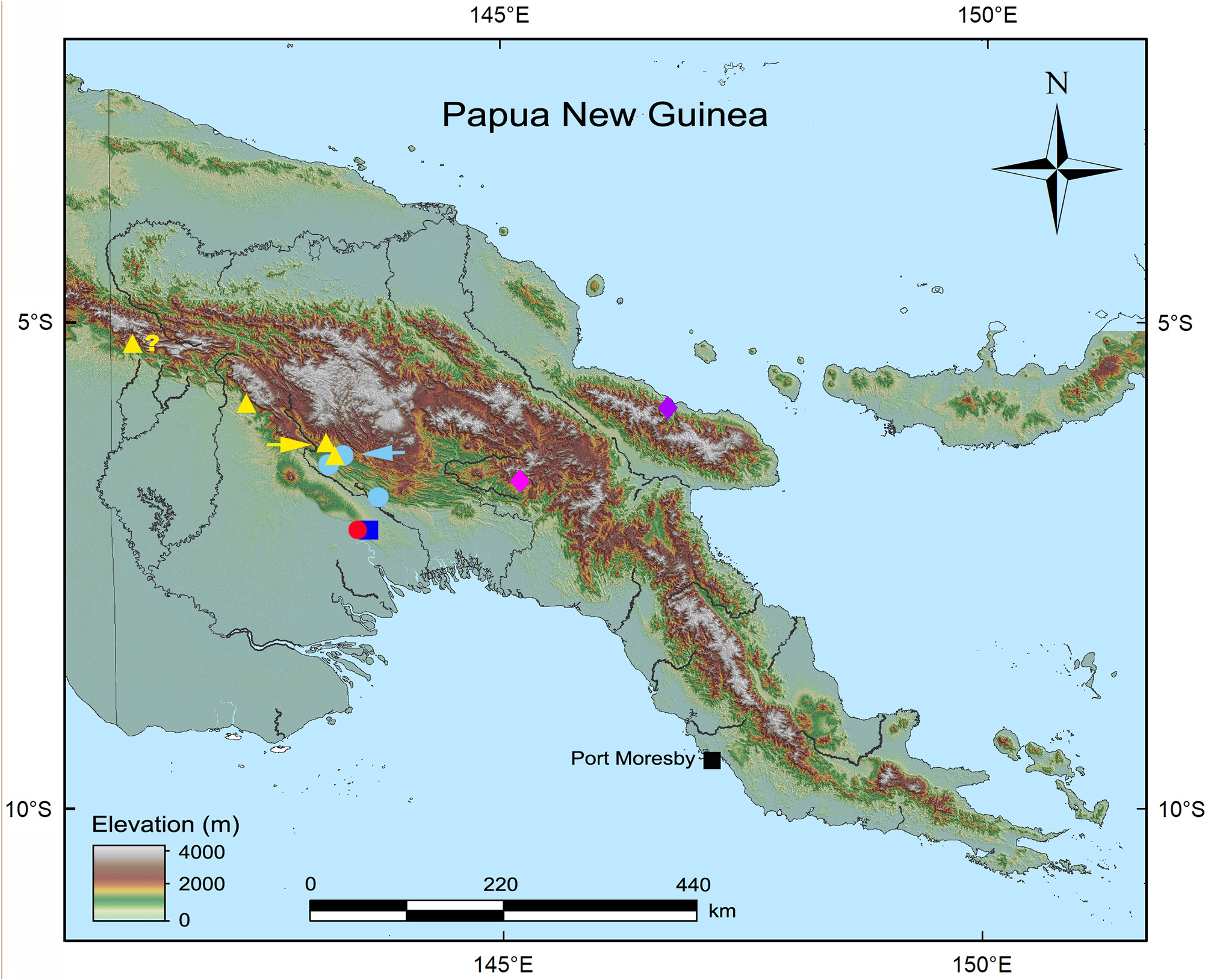
Distribution and ecology. Litoria gracilis sp. nov. is known from several sites across the southern foothills of Papua New Guinea’s Central Cordillera ( Fig. 4 View FIGURE 4 ) between the upper Strickland River basin in the west and the upper Kikori River basin in the east. The identity of frogs in the upper Fly River basin near the Indonesian border referred to L. nigropunctata by Hyndman & Menzies (1990) requires confirmation, but they may represent this species. We exclude the record of L. cf. nigropunctata from the southern foothills of the Snow Mountains in Papua Province, Indonesia ( Richards et al. 2015), pending genetic and acoustic analysis of that population.
All records of this species are from foothill rainforest where males call from perches on low foliage adjacent to, or overhanging, small, shallow streams and seepages ( Fig. 8 View FIGURE 8 ). We have observed adult males in close proximity to eggs and embryos attached to leaves over such waterbodies on several occasions ( Fig. 6E–F View FIGURE 6 ), including at the type locality on Iagifu Ridge. The only other pelodryadid frog observed at this location over many years (1999–2017) is a member of the Litoria genimaculata complex that deposits eggs directly into small streams, and we are confident that these ‘arboreal’ egg clutches belong to L. gracilis sp. nov. This conclusion is supported by the observation that the only known female of this species contains relatively large, yellow eggs typical of species that deposit their eggs on leaves above water (e.g., Menzies 1993). This reproductive strategy is an example of Mode 24 of Haddad & Prado (2005), although the small clear streams into which the tadpoles drop could arguably be considered lotic rather than lentic habitats.
IUCN Red List status. Litoria gracilis sp. nov. has a moderately broad distribution across more than 100 km of the southern foothills of Papua New Guinea’s Central Cordillera in an area where extensive tracts of primary forest remain and are likely to do so for the foreseeable future. We therefore recommend that it be assessed as Least Concern by the IUCN Red List.
Etymology. Gracilis is a Latin adjective meaning slender, graceful, or gracile, and refers to the slender body form of this species.
Molecular divergences. Based on analyses of a 787 base pair alignment from the mitochondrial ND4 gene and flanking tRNA L. gracilis sp. nov. is the sister taxon to L. daraiensis sp. nov. ( dA between the taxa of 0.08, Table 2 View TABLE 2 ). dA between sister species pairs in other groups of Litoria ranges from 0.04 to 0.25 ( Donnellan et al. 2021, Rowley et al. 2021). Samples of L. gracilis sp. nov. from different localities show moderate intraspecific divergence (maximum p-distance 0.052). Litoria gracilis sp. nov. was formerly confounded with L. nigropunctata (e.g., Richards 2003) but it is deeply divergent from topotypic L. nigropunctata from Yapen Island ( dA between the taxa of 0.15).
| SAMA |
South Australia Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.