Isodontia nigella (F. Smith, 1856 )
|
publication ID |
https://doi.org/10.25221/fee.481.3 |
|
publication LSID |
lsid:zoobank.org:pub:181E149D-3DFE-4777-9CC4-A0C1B8121466 |
|
persistent identifier |
https://treatment.plazi.org/id/03D48784-FFCF-FFC9-FF69-D343FF998D06 |
|
treatment provided by |
Felipe |
|
scientific name |
Isodontia nigella (F. Smith, 1856 ) |
| status |
|
Isodontia nigella (F. Smith, 1856) View in CoL
Figs 2–7
Sphex xanthognathus Pérez, 1905: 151 View in CoL , ♂ (type locality: “Yokohama” [ Japan]; holotype or syntypes in the Muséum National d’Histoire Naturelle, Paris, France). Synonymized with S. nigellus View in CoL by Berland, 1926: 283.
MATERIAL EXAMINED. Crimea, Karadag , 44°54′48″N 35°12′06″E, from nests, 12.VI–5.VII 2022, 22 ♀, 81 ♂, leg. A. Fateryga. GoogleMaps
DIAGNOSTIC CHARACTERS. Isodontia nigella can be easily distinguished from other European representatives of the genus. It differs from I. splendidula by the absence of a red pattern on the metasoma and from I. mexicana by the absence of a metallic shade of the body and the wings. Isodontia nigella differs from I. paludosa by a smaller body size and a longer and curved petiole of the metasoma (Figs 2, 4). Other diagnostic characters are illustrated in Figs 3, 5–7. In particular, the female propodeum is only slightly transversally wrinkled (Fig. 3) and the apical margin of the female clypeus has a remarkable narrow but deep incision at center (Fig. 6). The male clypeus is with indistinct incision at center of the apical margin (Fig. 7). The male genitalia are as in Fig. 5.
DISTRIBUTION. Russia: Crimea (new record), Primorsky Territory; China; Korean Peninsula; Japan; India; Australia.
Figs 2–7. Isodontia nigella (F. Smith, 1856) , ♀ (2, 3, 6) and ♂ (4, 5, 7): 2, 4 – habitus, lateral view; 3 – scutellum, metanotum, and propodeum, dorsal view; 5 – genitalia, dorsal view; 6, 7 – head, frontal view. Scale bars = 1 mm.
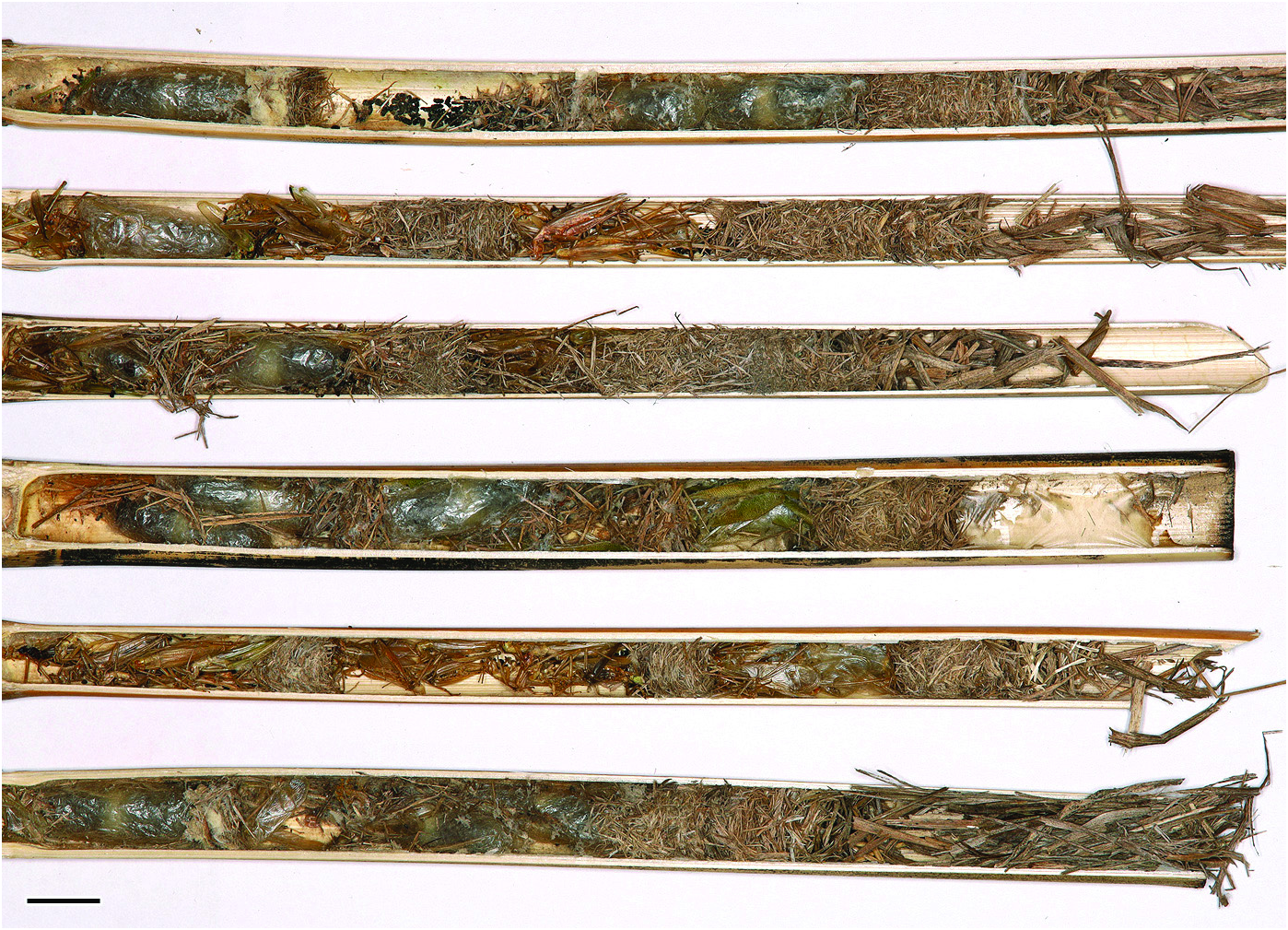
BIONOMICS. The nests of Isodontia nigella were situated in reed canes with the length of 7.2–29.2 cm (17.9 ± 1.2 cm on average; n = 73) and the inner diameter of 7.4–13.1 mm (9.7 ± 0.3 mm on average; n = 73). The partitions between the cells as well as the closing plug of the nest were made of packed fragments of grass stalks and blades ( Fig. 8 View Fig ). There were from one to eight cells per nest (2.5 ± 0.3 on average; n = 66). Some partitions between pairs of subsequent cells were absent so that two wasp cocoons appeared together in a “communal cell” ( Fig. 8 View Fig : the first, the fourth, and the sixth nests from above). Such “communal cells” with two cocoons were counted as two cells because it was uncertain if a flimsy partition between the provisions stored for each egg had existed initially, before the larvae finished feeding and started cocooning.
The prey of Isodontia nigella consisted of three species of orthopteran insects. They were found mainly in cells with dead wasp eggs (see below). In a few nests there were also “false cells” containing the provision only, without the wasp progeny. A total of 288 specimens of the prey were collected from 37 cells (including the four “false cells”). Among them, a tree cricket Oecanthus pellucens (Scopoli, 1763) ( Gryllidae : Oecanthinae) predominated with 238 imagines and 36 juveniles (95.1% of all identified specimens). The second prey species was a leaf katydid Phaneroptera nana Fieber, 1853 ( Tettigoniidae : Phaneropterinae) with two imagines and 11 juveniles and the third was Anadrymadusa retowskii (Adelung, 1907) ( Tettigoniidae : Tettigoniinae) with one juvenile specimen. There were from three to 15 victims stored per cell (8.6 ± 0.8 on average; n = 33).
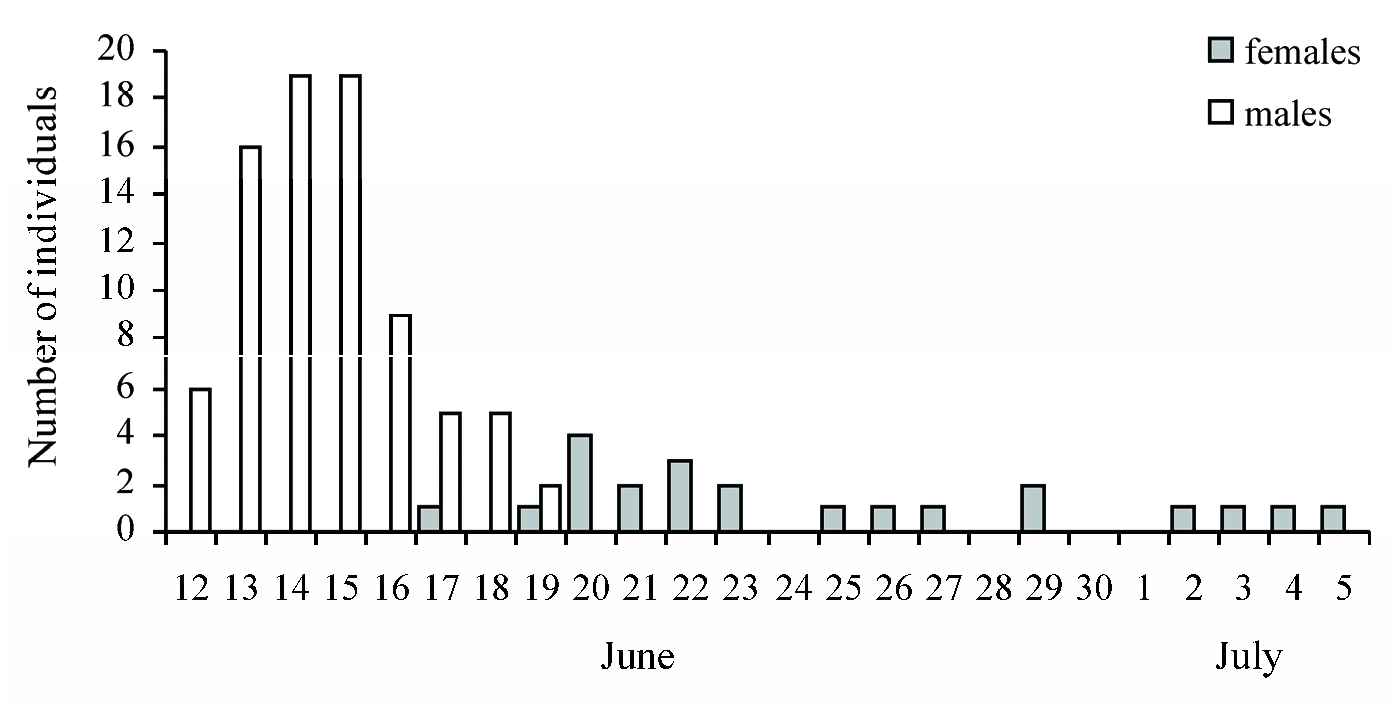
Among 73 nests, there were seven nests of the first generation with empty cocoons from which the wasps of the second generation had already emerged before 26 September 2021. The remaining 66 nests represented the nests of the second generation with the prepupae hibernating in the cocoons. Thus, the species had two generations per year. The progeny emerged from the nests of the second generation in 2022: males on 12–19 June, females on 17 June–5 July ( Fig. 9 View Fig ). A total of 103 specimens emerged: 22 females and 81 males. Thus, the sex ratio was strongly male-biased, about 1♀: 4♂. There were 51 nests from which at least one wasp emerged. Among them, nine nests produced females only, 11 nests produced imagines of both sexes, and 31 nests produced males only. In the nests with mixed progeny, females usually emerged from the inner cells while males emerged from the outer ones. There was the only exception in the largest nest with eight cells and the following sequences of sexes: ♂ ♀ ♂♂♂♂♂♂ (from the innermost cell to the outermost one).
Besides 103 successful cells (65.2% of a total of 158 cells in the nests of the second generation, without taking into account the “false cells”), there were 55 cells in which the progeny died. Two species of parasitoids were revealed in the nests: Melittobia acasta (Walker, 1839) ( Hymenoptera : Eulophidae ) in one cell and an unidentified bee fly ( Diptera : Bombyliidae ) in two cells. Most mortality cases were, however, for unknown cause, especially at the egg stage ( Table 1).
The record of Isodontia nigella from the Crimea is the first case of its introduction into Europe. This is the second invasive species of the genus Isodontia after I. mexicana which became established first in France in the early 1960s and finally reached Eastern Europe in 2000s and 2010s ( Ćetković et al., 2012; Fateryga et al., 2014; Amolin et al., 2018). Isodontia nigella is the fifth invasive species of Sphecidae in the Crimea and the eighth in Europe (see Schmid-Egger & Herb, 2018; Bitsch et al., 2020; Fateryga et al., 2020).
The results of the presented study of the nests of Isodontia nigella are largely consistent with the previously published data on this species ( Piel, 1933; Tsuneki, 1963, 1964; Barthélémy, 2012). Particularly, the prey of the genera Oecanthus Serville, 1831 and Phaneroptera Serville, 1831 were reported for this species from Japan ( Tsuneki, 1963). The same is true for the presence of several generations per year ( Tsuneki, 1963). A male-biased sex ratio in I. nigella was known in both Japan, 1♀:2.4 ♂ ( Tsuneki, 1964) and China, 1♀: 3♂ ( Barthélémy, 2012). It is of note that a male-biased sex ratio is typical of other species of Isodontia as well ( Barrett et al., 2021) while some species have rather equal sex ratio ( O’Neill & O’Neill, 2009).
Unusual cells with two cocoons have been also already recorded for Isodontia nigella by Tsuneki (1963). It is interesting that Gess & Gess (1982) reported cannibalism in the cells of I. pelopoeiformis in which two eggs had been deposited close to each other. It seems that a larva having devoured its first victim actively seeks out any conspecific larva in the cell and kills it. In the case of the presence of at least a flimsy partition between the provisions stored for each egg, the larvae are well separated spatially and cannibalism does not happen. In the case of I. nigella it was uncertain if a flimsy partition between the provisions stored for each egg had existed initially because both us and Tsuneki (1963) recorded “communal cells” at the cocoon stage only. It is possible that such partitions existed but were damaged by cocooning larvae. It is of note that Krombein (1967) speculated the evolution of the nest structure of the grass-carrying wasps from nests with substantial partitions between all cells, such as in I. elegans (F. Smith, 1856) , through nests with flimsy partitions, such as in I. mexicana , to nests with true communal brood chambers, such as in I. auripes . Obviously, I. nigella belongs to the second evolutionary stage, according to the Krombein’s (1967) hypothesis.
It is also of note that the reproductive success of Isodontia nigella in our study was relatively high in comparison with most native species of Sphecidae which usually had 28– 49% of successfully emerged progeny (Weawing, 1995; Fateryga & Kovblyuk, 2014; Barrett et al., 2021). Invasive species often have a higher reproductive success, about 64–67% ( Fateryga & Kovblyuk, 2013; Fateryga et al., 2020) that is similar to I. nigella . A high reproductive success of 62% was, however, reported for a native population of this species as well ( Barthélémy, 2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Isodontia nigella (F. Smith, 1856 )
| Fateryga, A. V., Ivanov, S. P., Mokrousov, M. V. & Fateryga, V. V. 2023 |
Sphex xanthognathus Pérez, 1905: 151
| Berland, L. 1926: 283 |
| Perez, J. 1905: 151 |
Sphex nigella F. Smith, 1856: 255 , ♀ ♂ (type locality: “ Shanghai ” [ China ]; syntypes in the British Museum of Natural History , London , U.K.)
| Smith, F. 1856: 255 |