Sudanonautes tiko Mvogo Ndongo, Schubart & Cumberlidge
|
publication ID |
https://doi.org/10.11646/zootaxa.4242.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:D18EDA2A-92BD-49A9-95A6-DA3F3CAC4336 |
|
DOI |
https://doi.org/10.5281/zenodo.5697098 |
|
persistent identifier |
https://treatment.plazi.org/id/03CF007B-FFCF-C42A-7FB6-2F5D1466FB69 |
|
treatment provided by |
Plazi |
|
scientific name |
Sudanonautes tiko Mvogo Ndongo, Schubart & Cumberlidge |
| status |
sp. nov. |
Sudanonautes tiko Mvogo Ndongo, Schubart & Cumberlidge View in CoL n. sp.
Common name: Tiko freshwater crab
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 , 6 View FIGURE 6 ).
Type material examined. CAMEROON. Tiko , Tamba Forest , Southwest Region ( 04° 10' 50''N, 09° 23' 40'E) 100 m asl, 1 adult ♂ holotype, CW 36.2 mm, CL 24.8 mm, CH 12.2 mm, FW 10.4 mm, 22 Aug. 2015 (P. A. Mvogo Ndongo) ( ZMB Crust. 29628; DNA extraction code T262-11). Edea, Mbus Michon , Littoral Region (03° 43' 146''N, 010° 7' 734''E), 137 m asl, adult paratype ♂, CW 35.4 mm, CL 25.1 mm, CH 12.0 mm, FW 10.3 mm, 10 Jul. 2015 (P. A. Mvogo Ndongo) ( ZMB Crust. 29629; DNA extraction code T262-7).
Other material examined. CAMEROON. Edea, Mbus Michon, Littoral Region (03° 43' 146''N, 010° 7' 734''E) 137 m asl, adult ♂, CW 28.69 mm, CL 20.71 mm, CH 9.65 mm, FW 9.16 mm, 9 Jul. 2015 (P. A. Mvogo Ndongo) (LZUY-01; DNA extraction code T262-8); Tiko , Bwenga forest , Southwest Region ( 04° 02' 52''N, 009° 19' 22'E), 38 m asl, adult ♂, CW 33.6 mm, CL 23.1 mm, CH 10.1 mm, FW 8.5mm, 24 Aug. 2015 (P. A. Mvogo Ndongo) ( RMNH. CRUST.D.57073; DNA extraction code T273-12).
Sudanonautes africanus . CAMEROON. Tiko , Bwenga forest , Southwest Region ( 04° 02' 52''N, 009° 19' 22'E), adult ♂, CW 92.8 mm, CL 68.6 mm , CH 27.6 mm, FW 25.9 mm 24 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-02); Tiko , Southwest Region ( 04° 02' 52''N, 009° 19' 22'E), 37 m asl, adult ♀, CW 87.4 mm, CL 66.0 mm , CH 26.9 mm, FW 23.5 mm, 26 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-03; DNA extraction code T273-10); Edea, Bolekoa stream , Littoral Region (03° 51' 288''N; 010° 4' 110''E), 115 m asl, 2 subadult ♂, largest CW 78.3 mm, CL 58.2 mm , CH 22.5 mm, FW 21.2 mm, 26 Nov. 2013 (P. A. Mvogo Ndongo (LZUY-04, DNA extraction code R1028-1); Edea, Bolekoa stream , Littoral Region (03° 51' 288''N; 010° 4' 110''E), 115 m asl, 2 subadult ♀, largest CW 62.6 mm, CL 47.2 mm , CH 18.7 mm, FW 16.2 mm, 26 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-05; DNA extraction code T263-4). Sudanonautes aubryi . CAMEROON. Edea, Bolekoa , Littoral Region (03° 51' 288''N, 010° 4' 110''E), 115 m asl, subadult ♂, CW 35.80 mm, CL 25.05 mm , CH 12.03 mm, FW 10.31 mm, 7 Jul. 2014 (P. A. Mvogo Ndongo) (LZUY-06; DNA extraction code R1026-9); Edea, Bolekoa , Littoral Region (03° 51' 288''N, 010° 4' 110'' E), 115 m asl, subadult ♀, CW 34.0 mm, CL 24.5 mm , CH 9.9 mm, FW 9.2 mm, 27 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-07; DNA extraction code T273-5); Didzangué , Island of Lake Ossa , Littoral Region ( 03°48.959' N, 010°03.309' E), 90 m asl, 2 subadult ♂ s, largest CW 32.7 mm, CL 23.8 mm GoogleMaps , CH 10.0 mm, FW 8.6 mm, 28 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-08; DNA extraction code: T262-9 and T262-10). Sudanonautes floweri . CAMEROON. Yabassi, Parc des Princes , Littoral Region ( 04°28.346''N, 009°57.433'E), 255 m asl, adult ♂, CW 35.38 mm, CL 24.93 mm GoogleMaps , CH 14.39 mm, FW 7.73 mm, 30 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-09); Yabassi, Parc des Princes , Littoral Region ( 04°28.346'N; 009°57.433'E), 255 m asl, adult ♀, CW 37.3 mm, CL 26.3 mm GoogleMaps , CH 14.1 mm, FW 9.1 mm, 30 Aug. 2015 (P. A. Mvogo Ndongo) (LZUY-10; DNA extraction code T262-3); Tiko , Tamba forest , Southwest Region ( 04° 10' 50''N, 009° 23' 40'E), 100 m asl, adult ♀, CW 53.88 mm, CL 38.0 mm , CH 20.4 mm, FW 11.7, 3 Sep. 2015 (P. A. Mvogo Ndongo) (LZUY-11); Tiko , Tamba forest , Southwest Region ( 04° 10' 50''N, 009° 23' 40'E), 100 m asl, adult ♂, CW 36.4 mm, CL 25.2 mm , CH 12.9 mm, FW 8.9 mm, 3 Sep. 2015 (P. A. Mvogo Ndongo) (LZUY-12; DNA extraction code T262-5).
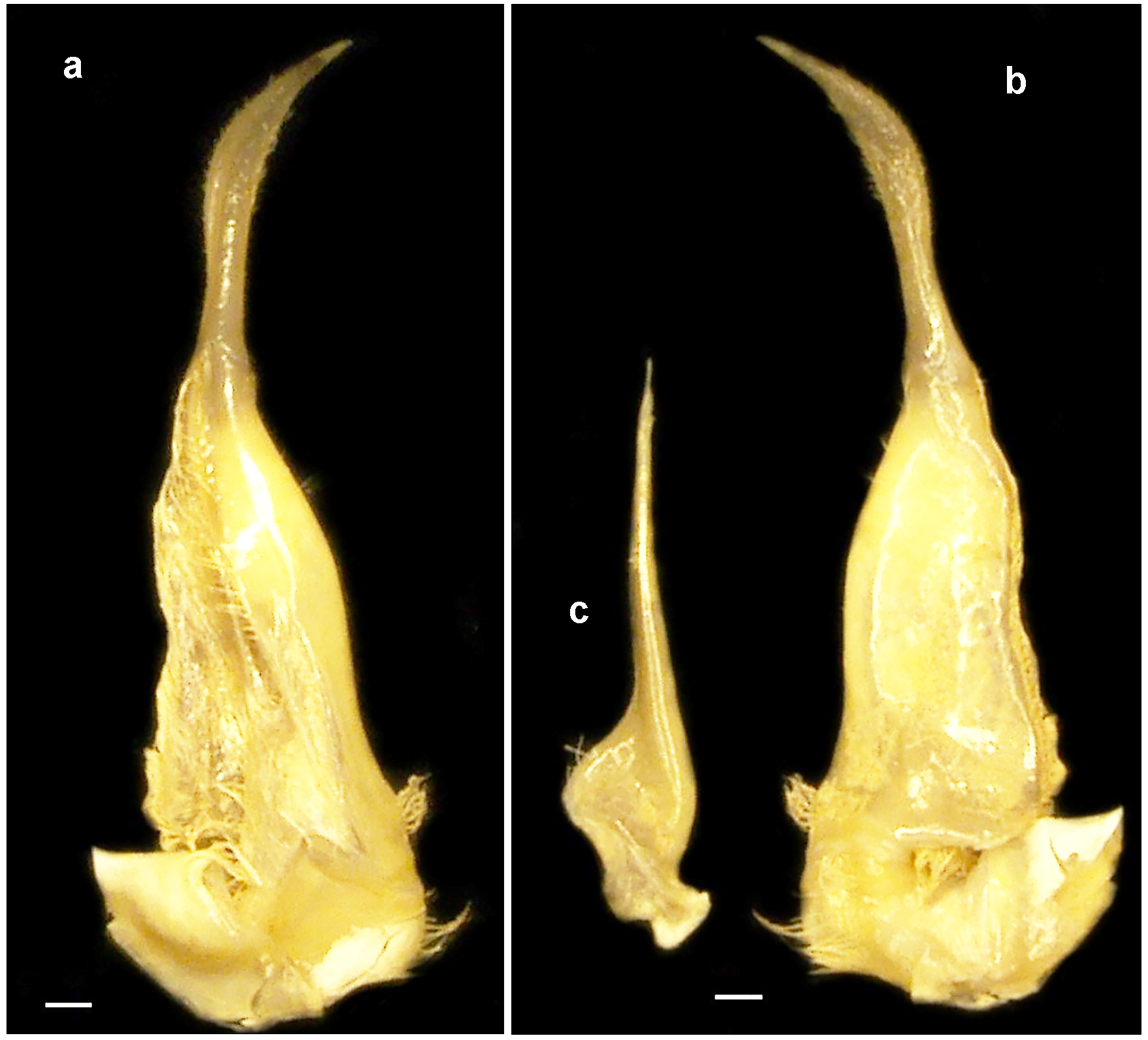
Diagnosis. Carapace subovoid; postfrontal crest distinct, completely crossing carapace, meeting epibranchial teeth; exorbital tooth low, blunt; exorbital tooth reduced to granule; intermediate tooth between exorbital, epibranchial teeth present, reduced to granule ( Fig. 1 View FIGURE 1 a,b, 2a,b). Vertical sulcus on carapace sidewall meeting anterolateral margin at epibranchial tooth ( Fig. 2 View FIGURE 2 a,b). Third maxilliped ischium with distinct vertical sulcus ( Fig. 2 View FIGURE 2 d). S3/s4 reduced to 2 short notches on lateral margins; margins of s1–s4 distinctly thickened, raised; anterior margin of sternoabdominal cavity low, not raised ( Fig. 2 View FIGURE 2 c). Fingers of male major cheliped slim, elongated, fixed finger (pollex) with 2 large teeth proximally, movable finger (dactylus) arched, with single large tooth one-third distance from base, enclosing oval interspace when closed ( Fig. 2 View FIGURE 2 f); cheliped carpus with 2 acute teeth, distal tooth larger than proximal ( Fig. 1 View FIGURE 1 a,b). G1 subterminal article slim; G1 terminal article long, one third length of gonopod, proximal G1 terminal article straight basally, distal half curving sharply outward, widened medially, evenly tapering to narrow, pointed tip ( Fig. 3 View FIGURE 3 a,b). Terminal article G2 extremely short, only one-fifteenth as long as subterminal segment ( Fig. 3 View FIGURE 3 c).
Description. Male holotype. Carapace subovoid, widest at anterior third (CW/CL 1.46), high ( CH /CL 0.49), front broad (FW/CW 0. 29), deflexed, anterior margin slightly concave medially; postfrontal crest distinct, complete, consisting of fused epigastric, postorbital crests, divided medially by shallow median sulcus forked posteriorly, each lateral end curving back to meet epibranchial tooth; urogastric, cardiac, cervical grooves all distinct; exorbital tooth short, blunt; anterolateral margin between exorbital, epibranchial teeth curving slightly outward, with small granule-sized intermediate tooth; anterolateral margin smooth posterior to epibranchial tooth ( Figs. 1 View FIGURE 1 a,b, 2a,b); suborbital margin smooth; epistomial median tooth large, triangular, edges lined by granules ( Fig. 2 View FIGURE 2 c). Carapace sidewall with 2 sutures, 1 longitudinal, 1 vertical, dividing sidewall into 3 parts ( Fig. 2 View FIGURE 2 a,b); longitudinal suture dividing suborbital, subhepatic regions from pterygostomial region, beginning at respiratory opening, curving backward across side wall; short vertical suture dividing suborbital region from subhepatic region; additional suture beginning at base of minute epibranchial tooth, curving forward under intermediate tooth, then curving sharply down meeting longitudinal suture, marked by row of granules. S2/s3, completely crossing sternum; s3/s4 incomplete, reduced to one small notch at each side ( Fig. 2 View FIGURE 2 c). Third maxillipeds filling entire buccal cavern except for transversely oval efferent respiratory openings visible at superior lateral corners ( Fig. 2 View FIGURE 2 c); exopod with well-developed slender flagellum; ischium with distinct vertical sulcus ( Fig. 2 View FIGURE 2 c).
Chelipeds of adult male unequal, right (major) cheliped (propodal length 53.3 mm) 1.3 times longer than left cheliped (propodal length 42.8 mm). Fingers slim, elongated; movable finger (dactylus) arched, with single large tooth one-third distance from base, enclosing oval interspace when closed; fixed finger with 2 large teeth proximally. Minor cheliped with occluding margins of fingers lined by row of small teeth ( Fig. 1 View FIGURE 1 a,b); carpus with 2 sharp teeth, distal tooth larger than proximal; inferior margins of merus bearing series of small sharp teeth and a larger distal edge tooth ( Fig. 2 View FIGURE 2 e), distal tooth sharp, superior surface of merus smooth ( Fig. 2 View FIGURE 2 e). Walking legs (pereiopods 2–5) moderately slender, posterior margins of propodi serrated with small blunt teeth, dactyli tapering, each bearing rows of downward-pointing large sharp spines ( Fig. 1 View FIGURE 1 a,b).
Male pleon (abdomen) broadly triangular with straight edges ( Fig. 2 View FIGURE 2 c). G1 terminal article long (about onethird length of total length), proximal half straight, distal half curving sharply outward, widened medially, with short setae, evenly tapering to narrow, pointed tip ( Fig. 3 View FIGURE 3 a,b); G2 shorter than G1 reaching only to G1 subterminal segment/terminal article junction; terminal article extremely short, only one-fifteenth as long as subterminal segment; G2 subterminal segment widest at base, then tapering sharply inward, forming long, thin, pointed, upright process supporting short terminal segment; rounded collar at junction between terminal article and subterminal article ( Fig. 3 View FIGURE 3 c).
Size. A medium-sized species, adult at CW 36.2 mm.
Color in life. Holotype from Tiko ( Fig. 1 View FIGURE 1 a) dorsal carapace and appendages dark brown, green in specimens from Edea ( Fig. 1 View FIGURE 1 b); articulations between carpus and merus of cheliped and ambulatory legs pale yellow-brown. Preserved specimens uniformly light brown.
Type locality. Cameroon, Tamba Forest , Tiko , in the Southwest Region ( 04°10'50''N, 09°23'40'E), 100 m asl.
Etymology. The new species is named for Tiko in southwestern Cameroon, where it was first collected. The species name tiko is a noun in apposition.
Habitat. Sudanonautes tiko n. sp. is restricted to the humid area of the coastal rain forest of Cameroon. Tiko (the type locality) and Edea both lie in the coastal zone of Cameroon which is one of the wettest places in Africa, with an annual average rainfall of more than 5,000 mm, which includes Mount Cameroon ( 4,095 m asl), the highest mountain in West and Central Africa ( Molua 2009). At Edea, S. tiko n. sp. was found under small stones in wetlands near the Sanaga River ( Fig. 4 View FIGURE 4 ) and not in burrows, while at Tiko the new species was found in small streams under small rocks. At the Tiko locality, S. tiko n. sp. occurs sympatrically in the streams with other species of freshwater crabs such as S. africanus and S. floweri .
Sudanonautes tiko View in CoL n. sp. may be threatened by human impacts on its habitat. The water in the streams at Tiko View in CoL was found to be quite acidic (between pH 4.0–6.5) and at a lower pH than would normally be expected for a tropical freshwater ecosystem (where the majority of aquatic organisms prefer water with a pH between 6.5–9.0 (Robertson-Brayan 2004)). The lower pH range of the water at Tiko View in CoL may be attributed to anthropogenic pollution from human wastes. Changes in key physical and chemical parameters at the landscape scale ( Walsh et al. 2005; Callaghan et al. 2005; Prowse et al. 2006) are likely to affect the species richness and biodiversity freshwater ecosystems and to alter the food webs and the levels of primary and secondary productivity. In contrast, the pH of the stream at Edea was less acidic and was in the expected pH range (6.5–9.0). The populations of S. tiko View in CoL from Edea, however, may be threatened by the intensive and destructive agricultural practices that are already impacting the condition of the Sanaga River, which have the potential to extirpate local populations of the freshwater crabs that depend on the ecosystem for their existence.
Distribution. Sudanonautes tiko n. sp., is so far known only from lowland tropical rainforest in the Northern and Southern gulfs of Guinea drainage ecoregions where the species is associated with two major rivers close to where they drain into the ocean. The localities where this is known to occur are Tiko , Tamba Forest and Bwenga Forest in the Mungo River Basin, close to Mount Cameroon, Southwest Region, and Edea in the Littoral Region of Cameroon in the Sanaga River drainage close to the main river. The Mungo and Sanaga rivers both drain into the Atlantic Ocean in the Bay of Benin, and the above localities are all in tropical rainforest. Three freshwater ecoregions are represented in southern part of Cameroon where this study was carried out ( Thieme et al. 2005; Abell et al. 2008), and all have endemic species of freshwater crabs. These ecoregions are: (1) the Northern Gulf of Guinea drainage in Cameroon (where S. tiko n. sp. is found with Louisea balssi , Potamonemus mambilorum , and S. orthostylis ); (2) the Southern Gulf of Guinea drainage in southern Cameroon (where S. tiko is found with L. edeaensi s); (3) and the Sangha ecoregion, which includes the rivers and streams of the lowland forests of southeast Cameroon, Congo, and western Central African Republic (where S. sangha and Potamonautes regnieri are endemic).
Remarks. The new species is assigned to Sudanonautes because it conforms to the genus in every diagnostic aspect ( Bott 1955; Cumberlidge 1999). Sudanonautes tiko n. sp. can be distinguished from all other congeneric species by the following characters: the G1 terminal article of S. tiko is conspicuously long (about one-third the length of the entire gonopod), whereas in all other species of this genus the G1 terminal article is only about onequarter of the length of the entire gonopod. Other distinctions between S. tiko n. sp. and its congeners are as follows: the new species can be distinguished from S. africanus , S. chavanesii , S. faradjensis , and S. sangha (the four large-bodied species in this genus that are found in Cameroon and Central Africa) by its medium body size with a pubertal moult around CW 35 mm (vs. a pubertal moult at CW 50 mm or greater in these other four species). Sudanonautes tiko n. sp. can be distinguished from S. aubryi and S. granulatus on the basis of the size of the intermediate tooth on the anterolateral margin, which is reduced to a small granule in S. tiko n. sp. (vs. a large, triangular tooth in these two species). Sudanonautes tiko n. sp. can be distinguished from S. monodi and S. floweri by the width of the subterminal segment of G1, which is slim in S. tiko n. sp. (vs. distinctly widened in these two species). Finally, S. tiko n. sp. can be distinguished from S. kagoroensis from central Nigeria by the distinctly thickened outer margins of sternal segment s 4 in S. tiko n. sp. (vs. smooth and not thickened in S. kagoroensis (see Cumberlidge 1999)).
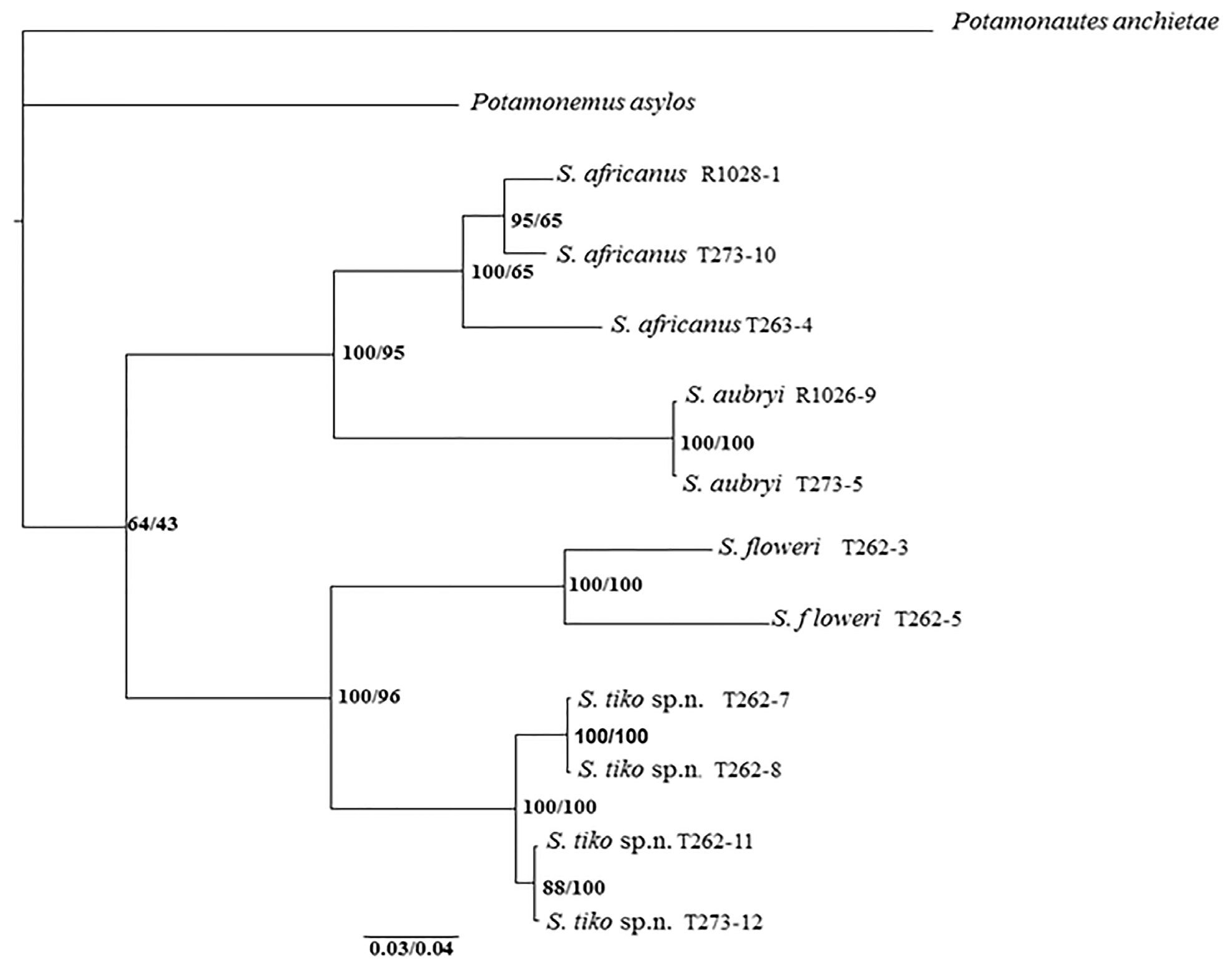
Molecular analysis. The phylogenetic tree with confidence values according to BI and ML derived from 1475 basepairs of combined COI and 16S RNA mitochondrial DNA sequences ( Fig. 5 View FIGURE 5 ) recovered all species of Sudanonautes as a single clade, but with relatively low confidence values (BI and ML values of 64/43 at this node, respectively). Each of the included Cameroonian species (i.e., S. africanus , S. aubryi , S. floweri , and S. tiko n. sp.) were all found to be well-supported independent lineages. Sudanonautes tiko n. sp. and S. floweri were grouped together as sister species by a node with strong support (BI/ML: 100/96), as were S. africanus and S. aubryi (BI/ ML: 100/95).
The phylogenetic tree offers insights into the large amount of genetic diversity within each of the species of Sudanonautes from the coastal zone of Cameroon. For example, populations of S. tiko n. sp. from Tiko in the Southwest Region form a separate subgroup within the clade from populations of this species from Edea in the Littoral Region. A considerable amount of genetic diversification was also found between populations of S. africanus from Tiko (T273-10) and Edea (T263-4). This species is nevertheless supported as a monophyletic taxon (BI/ML: 100/65).
Ecological Comparisons. Sudanonautes africanus ( Fig. 6 View FIGURE 6 a) occurs in the coastal rainforest regions of Nigeria and Central Africa ( Cumberlidge 1995c, 1999) and was collected during the present study from rainforest localities along the coastal region between Mount Cameroon and the border with Equatorial Guinea (Campo, Kribi, Bipindi, Lake Ossa, Edea, Yabassi, Tiko ). This species has also been collected from a number of other localities in Cameroon ( Lake Barombi, Kumba, Limbe, Yaounde, Enongal, and Ebolowa; Cumberlidge 1999). Sudanonautes africanus was the commonest freshwater crab collected during this study and its abundance and large size make it a popular food item for the local population. This species occurs in a range of permanent aquatic habitats from large rivers to small streams ( Cumberlidge 1999). Male and female sub-adults and juveniles were captured during the day either by hand from under stones in small streams or from large rivers downstream of the collection site, if they escaped in the turbid waters created when the rocks were turned. Some were trapped at night entangled in nylon fishing nets set bank-to-bank across the stream and baited with cassava. Others were collected in a sieve passed through the water. These methods tend to capture only juveniles, because the larger subadults and adults of S. africanus are semiterrestrial, live in 1 m deep burrows close to streams, and need to be collected by hand or caught at night in pitfall traps ( Cumberlidge 1999). Adult males and females of S. africanus dig separate burrows and do not co-inhabit a single hole. The burrows dug by adult males were further away from the water than those of adult females and subadults of both sexes. The distribution of the size classes in the population indicates that large adult specimens of S. africanus are less abundant than the more numerous smaller individuals ( Cumberlidge 1999), so that the capture of reproductive adults causes the greatest loss to the population. In addition, juveniles and subadults of S. africanus living in the coastal zone of Cameroon are heavily exploited as a food source by local human populations. These food harvesting activities constitute a serious threat to the long-term existence of this species in this part of Cameroon. The carapace, claws and walking legs of S. africanus are dark brown in life.
Sudanonautes aubryi View in CoL ( Fig. 6 View FIGURE 6 b) is found both in the Lower Guinea and Upper Guinea forest zones in the rainforest and moist guinea savannas in Côte-d'Ivoire, Ghana, Togo, Benin, Nigeria View in CoL , Cameroon, Equatorial Guinea, and Gabon ( Cumberlidge 1999). In Cameroon, this species was collected from rainforest localities along the coastal region near Edea. Sudanonautes aubryi View in CoL has also been collected from the following localities in Cameroon: Lake Barombi, Kumba, Limbe, Yaounde, Bipindi, Enongal, and Ebolowa, and in the Mfiende, Enyumu, and Lokoundié Rivers ( Cumberlidge 1999). All size classes of S. aubryi View in CoL were collected from streams and rivers, and none were collected on land or in burrows near the water, and this species was sympatric with juveniles of S. africanus View in CoL . Sudanonautes aubryi View in CoL was collected either by hand or with a nylon fishnet or dipnet from under rocks and stones in the stream bed, and was only found in this study in two localities near Edea (Bolekoa stream and Bedimet Island in Lake Ossa). The carapace, claws and walking legs of S. aubryi View in CoL are generally yellowish brown in life.
Sudanonautes floweri View in CoL ( Fig. 6 View FIGURE 6 c,d) is found in the moister regions of the woodland and guinea savanna zones from central Nigeria View in CoL to South Sudan and northern Uganda and from humid tropical rainforest habitats in Cameroon, Bioko, Central African Republic, D. R. Congo, Congo, Gabon, Cabinda Angola, and Equatorial Guinea ( Cumberlidge 1999). In Cameroon, this species was collected only in rainforest habitats at Yabassi in south Cameroon, and at Tiko View in CoL near Mount Cameroon, but it has also been recorded by others from Limbe and Bibundi west of Mount Cameroon ( Cumberlidge 1999). Sudanonautes floweri View in CoL is found under rocks in shallow streams, rivers, and ponds, in burrows near waterways, and on land next some distance from the nearest water ( Cumberlidge 1999). At Yabassi, S. floweri View in CoL digs burrows near streams, whereas at Tiko View in CoL it is found in the stream together with S. africanus View in CoL and S. tiko View in CoL n. sp.. It is noteworthy that males and females of S. floweri View in CoL usually share the same burrow (in contrast to S. africanus View in CoL , where adult males and females dig separate holes). The carapace of S. floweri View in CoL is either dark brown, yellowish, or purple, and the claws and walking legs are pale yellow in life. At Tamba forest in Tiko View in CoL S. floweri View in CoL is sympatric (living in the same stream) with S. tiko View in CoL n. sp. and S. africanus View in CoL .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Potamonautinae |
|
Genus |