Isoodon auratus (Ramsay, 1887)
|
publication ID |
https://doi.org/10.5281/zenodo.6621742 |
|
DOI |
https://doi.org/10.5281/zenodo.6620286 |
|
persistent identifier |
https://treatment.plazi.org/id/03C91729-FFD7-FFB5-F86C-D9D3FCB41A01 |
|
treatment provided by |
Felipe |
|
scientific name |
Isoodon auratus |
| status |
|
2. View On
Golden Bandicoot
French: Bandicoot doré / German: Goldbrauner Kurznasenbeutler / Spanish: Bandicut de hocico corto dorado
Other common names: Northern Golden Bandicoot, Northern Golden-backed Bandicoot
Taxonomy. Perameles auratus [sic] Ramsay, 1887 ,
“ Derby , N. W. Australia.” (= Western Australia, Australia).
Recent molecular analyses confirm this taxon as being distinct from I. obesulus and distinguish two forms within it that correspond to subspecies auratus and barrowensis. As noted above, the same analyses suggest that these subspecies cluster with I. o. peninsulae (or even I. peninsulae), and further work may see this form grouped within the “Golden Bandicoot clade.” A further race, arnhemensis, has been distinguished on basis ofits larger size and slight differences in shape ofits skull; it has not, however, been subject to any genetic or molecular analysis, and is not accepted by all authorities. Three subspecies tentatively recognized here.
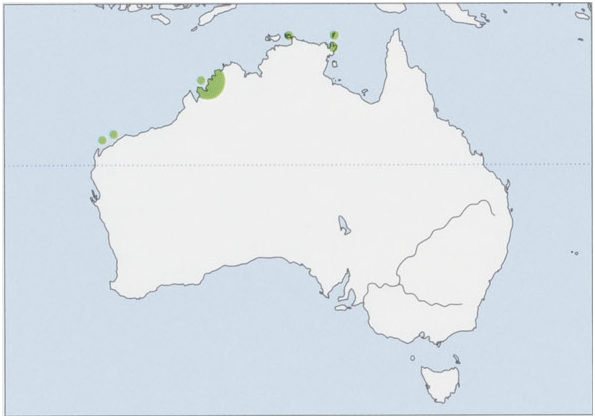
Subspecies and Distribution.
I.a.auratusRamsay,1887—NWKimberleyandseveralsmallislandsoffKimberleycoast.
I.a.arnhemensisLyne&Mort,1981—CapeArnhem,NENorthernTerritory.
I. a. barrowensis Thomas, 1901 — Barrow I and Middle I, Western Australia.
Occurs also (subspecies uncertain) on Marchinbar I, in Wessel Group, Northern Territory, and introduced recently to two further islands in Wessel Group. Barrow I race barrowensis introduced to nearby Hermite I, to Doole I (in Exmouth Gulf), and to Lorna Glen (mainland of C Western Australia) in recentyears. View Figure
Descriptive notes. Head-body 19-29.5 cm, tail 8.4-12.1 cm; weight 300-670 g for I. a. auratus , 250-600 g for I. a. barrowensis. Males 40-50% heavier than females. Smallest member of genus. Dorsal fur is golden brown, but has grizzled appearance owing to overlay of long, stiff guard hairs; ventral fur is cream or white. Tail is covered in short, orange to dark brown hairs.
Habitat. Prior to its final disappearance from arid regions in mid-20" century, occurred on sandy soils dominated by hummock grass or low shrubs. In its remnant mainland distribution it occurs in tussock grassland and low woodland dominated by Acacia (Fabaceae) and Eucalyptus spp. (Myrtaceae) , often on sandy soils, but also on rugged sandstone habitats. Shows a strong association with hummock grass on Barrow Island, often burrowing under hummocks for shelter, but on Marchinbar Island prefers low heath or shrubland on sand or sandstone. Sometimes found in vine thicket and patches of rainforest, but these do not appear to be preferred habitats. Nest can be a shallow scrape under rock or other shelter, or simple structure constructed from grass and other local materials; constructed on ground surface, under grass hummock and dense shrubs or, on Barrow, in limestone cave. Individuals use different shelters on different days, and visit varied habitats by night when foraging, but generally occupy stable ranges that change little between seasons.
Food and Feeding. Invertebrates represent a larger part of diet compared with those of both larger congeners, with beetles, termites, ants, centipedes, and insect larvae consumed. In addition, it eats seeds, roots, tubers, fruits, and other plant material, especially in dry season, and small rodents and lizards also may be eaten. Perhaps because of the diverse habitats that it uses for foraging, including ocean beaches, this species includes in its diet items that are unusual for peramelids, such as washed-up cephalopods and turtle eggs. Conical-shaped pits in sandy soils indicate where foraging has taken place.
Breeding. Females reproduce throughout year in all localities where repeated sampling has been carried out, but proportion of females giving birth increases during wet season in summer or after significant rain at other times. Rainfall probably increases availability of food for this species, and also provides free water that assists mothers in maintaining water balance during lactation. Females have eight nipples in a rearopening pouch and carry 2-3 young at a time. One or two young may survive to weaning, but rate of recruitment to adult population is not known. Age at sexual maturity is 6-7 months. There is no information for this species on length of gestation or time to weaning, but these traits are likely to be similar to those of better-studied Southern Brown Bandicoot ( 1. obesulus ).
Activity patterns. Rests by day in a nest or temporary shelter and emerges after dusk when conditions are dark. Radio-tracking studies indicate that activity can be maintained continuously throughout the night, although animals are usually most active 3—4 hours after dusk and in the hour or so before dawn. Up to 10 ha may be covered each night, although 1-5 ha is more usual. Animals return to their shelters before daybreak.
Movements, Home range and Social organization. Limited research on Barrow Island and Marchinbar Island shows that males occupy larger home ranges (4-4-35-6 ha) than females (1-7-12-7 ha). On Marchinbar, home range areas tended to be larger during dry season than in wet season and ranges of the sexes overlapped. On Barrow, male ranges overlapped with those of females but not with each other. Individuals appear to be generally aggressive toward each other and fight if placed in close proximity. Despite this social intolerance, population densities can be high. Marchinbar supports ¢.0-07 ind/ha and Barrow c.0-3 ind/ha, but density may rise to 10 ind/ha in productive areas on the coast, where island food resources are high and subsidies are available from nearby beaches.
Status and Conservation. Classified as Vulnerable on The IUCN Red List. This species was once widespread in interior of Australia, but is now confined to high-rainfall areas of north-western Kimberley (including Augustus and Uwins islands), Barrow Island and Middle Island, off Pilbara coast, all in Western Australia, with an isolated outpost on Marchinbar Island, off N Northern Territory. It is considered to have a declining population trend. Sparsely distributed on mainland Western Australia, where population declines recently recorded. No estimates available for Augustus or Uwins, but the species is said to be sparse on former and relatively common on latter. It appears to be secure on Barrow, where at least 20,000 individuals estimated, and reasonably safe on Middle (c.1000) and Marchinbar (c.1400). Introductions or reintroductions from these populations to two small Wessel Group islands (Guluwuru and Raragala islands) off N Northern Territory, and to Doole Island, Hermite Island, and Lorna Glen in Western Australia, should provide insurance that these forms will remain secure. These sites are conservation reserves or Indigenous Protected Areas and are monitored on a regular or ad hoc basis. Mainland populations of this bandicoot continue to decline owing to predation from domestic or feral cats (Felis catus), habitat loss due to such practices as mining, and inappropriate changes to the fire regime that reduce vegetation cover and expose animals to increased risk of predation. Monitoring takes place at some mainland sites such as Mitchell Plateau and Artesian Range Sanctuary, in Western Australia, with active research and management in place at latter site to mitigate effects of changed fire regimes. Long-term security of this peramelid most likely to occurif cats and other invasive species, such as the Roof Rat (Rattus rattus), are prevented from establishing on key islands, and if means of managing cat impacts on a broad scale can be found for the remnant populations that persist in mainland areas.
Bibliography. Bradshaw et al. (1994), Burbidge et al. (1988), Close et al. (1990), Cockburn (1990), Dahl (1897), Ellis et al. (1991), Friend, J.A. (1990a), Groves (2005c¢), Johnson & Southgate (1990), Jones et al. (2009), Lyne & Mort (1981), McKenzie, Morris & Dickman (2008), Ottewell et al. (2014), Palmer et al. (2003), Pope et al. (2001), Southgate et al. (1996), Westerman et al. (2012), Woinarski, Burbidge & Harrison (2014d), Woinarski, Palmer et al. (1999), Woinarski, Pavey et al. (2007), Zenger et al. (2005).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |
Isoodon auratus
| Russell A. Mittermeier & Don E. Wilson 2015 |
Perameles auratus [sic]
| Ramsay 1887 |