Prosthiostomum torquatum, Tsuyuki & Oya & Kajihara, 2019
|
publication ID |
https://doi.org/ 10.12782/specdiv.24.137 |
|
publication LSID |
lsid:zoobank.org:pub:BA5704AF-2761-4E17-B9EF-65506E3E33B1 |
|
persistent identifier |
https://treatment.plazi.org/id/B3572124-25E8-451D-930D-2363C19C6AB8 |
|
taxon LSID |
lsid:zoobank.org:act:B3572124-25E8-451D-930D-2363C19C6AB8 |
|
treatment provided by |
Felipe |
|
scientific name |
Prosthiostomum torquatum |
| status |
sp. nov. |
Prosthiostomum torquatum sp. nov. ( Figs 1–3 View Fig View Fig View Fig )
Material examined. Holotype: ICHUM 5563 View Materials , 13 View Materials slides ( HE), collected by Y. Oya in Shirahama (33.6926°N, 135.3332°E), Wakayama, Japan, on 22 August 2017 GoogleMaps . Paratypes (4 specimens, all from the type locality): ICHUM 5562 View Materials , sections through reproductive structures (7 slides) and anterior part (4 slides) ( HE), collected by N . Jimi on 16 May 2015; ICHUM 5565 View Materials , 9 View Materials slides ( HE), collection data same as holotype; ICHUM 5566 View Materials , 12 View Materials slides ( HE), collection data same as holotype; ICHUM 5567 View Materials , 13 View Materials slides ( MT), collected by Y GoogleMaps . Oya in Shirahama (33.6951°N, 135.3440°E), Wakayama, Japan, on 23 August 2017 GoogleMaps . Non-type specimen: ICHUM 5564 View Materials , unsectioned, preserved in 70% ethanol, collection data same as holotype GoogleMaps .
For comparison, we also examined digital photomicrographs of the male copulatory apparatus in the holotype ( AM W. 44692, MI QLD 2395 ) and paratype ( AM W.44065, MI QLD 2351 ) of Lurymare clavocapitata deposited at the Australian Museum, Sydney .
Type locality. Shirahama, Wakayama, Japan .
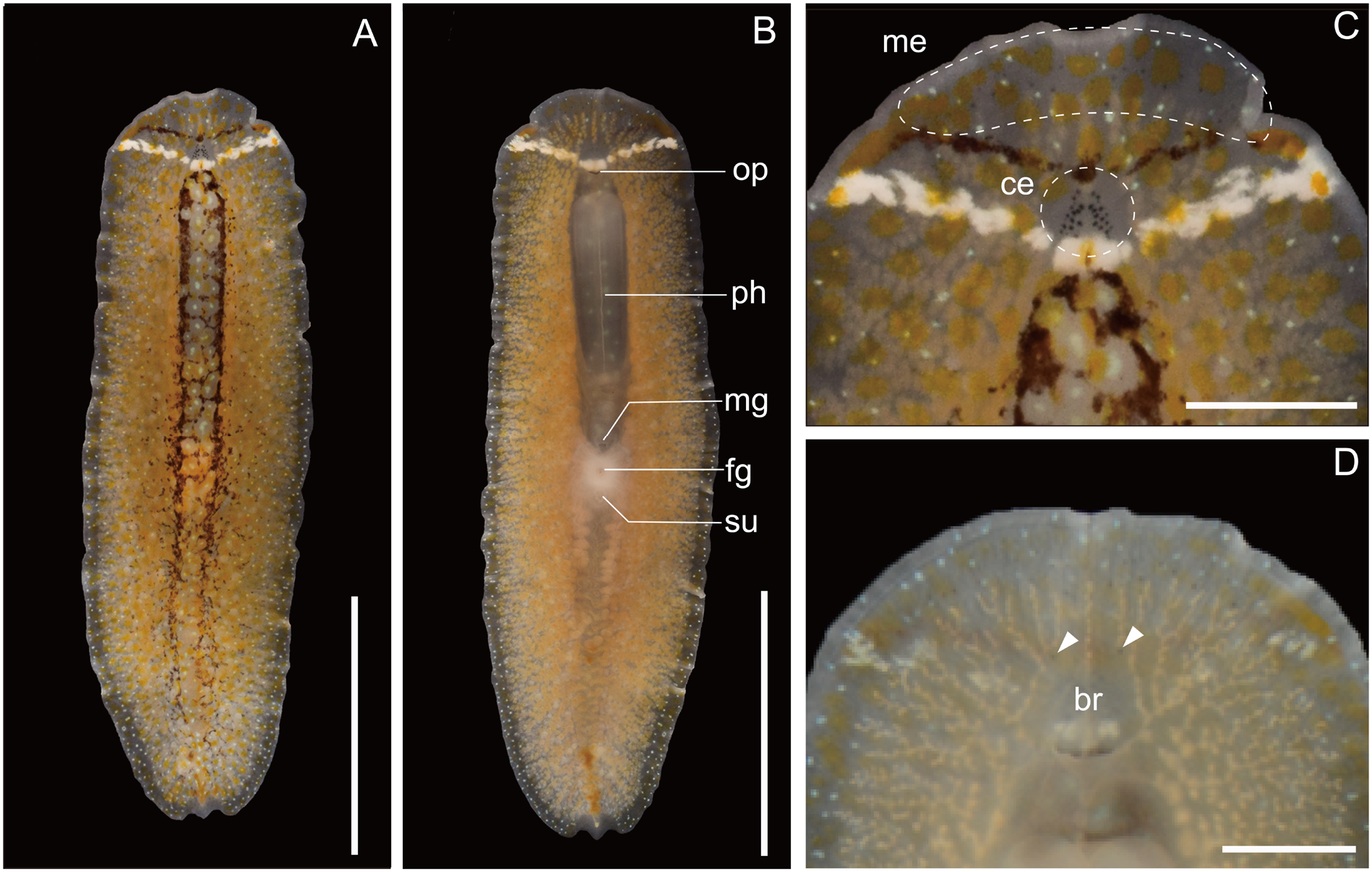
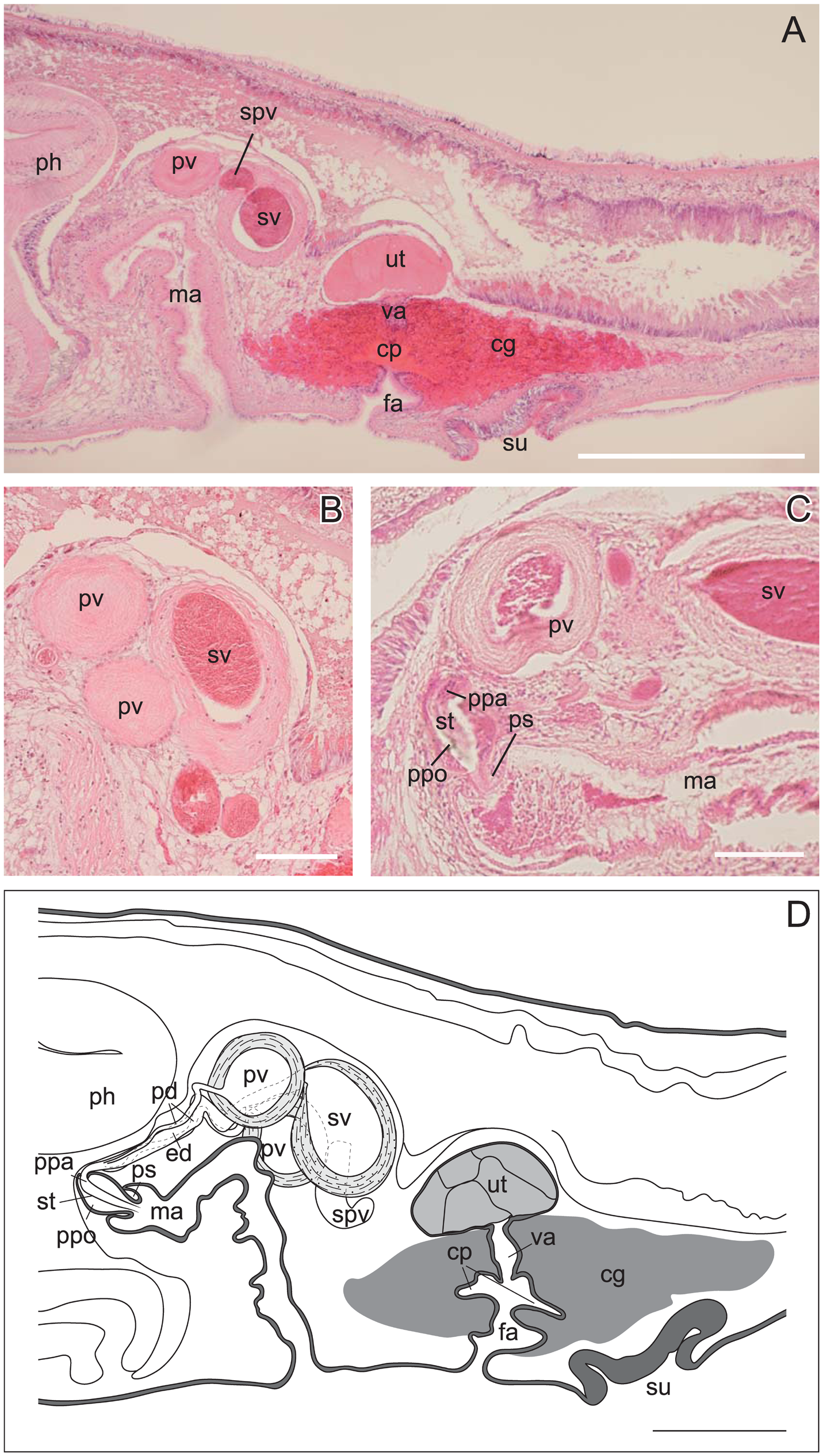
Description. Body elongated, anterior margin rounded, slightly tapered posteriorly, mid-point of posterior margin acute, 9–18 mm long (14 mm in holotype) and 2.5–5 mm maximum width (4 mm in holotype) in living state ( Fig. 1A, B View Fig ). Tentacles absent. Dorsal surface smooth, translucent, covered with numerous orange maculae and blue dots; orange maculae denser medially, blue dots uniformly scattered; each orange macula larger than single blue dot. Single transverse narrow dark-brown line running on dorsal surface of body in front of cerebral eyespots; its mid-point slightly arched backwards. Another transverse white line on dorsal surface, situated in short distance behind dark-brown line, likewise bending posteriorly at mid-point. Dark-brown pigments aggregating mid-dorsally to form incomplete, mesh-like, posteriorly-fading band, which runs from behind transverse white line ( Fig. 1A View Fig ). Body margin transparent. Ventral surface transluscent, without colour pattern. Intestine highly branched, spreading all over body. Pair of cerebral-eyespot clusters situated between transverse dark- brown and white lines near midline, each consisting of 7–13 eyespots (13 eyespots each in holotype); cerebral-eyespot clusters medially approaching each other at two or three points; two size categories among eyespots, larger one being more than twice the size of smaller one ( Fig. 1C View Fig ). Marginal eyespots sparsely anterior to transverse dark-brown line; marginal eyespots smaller than cerebral ones ( Fig. 1C View Fig ). Pair of ventral eyespots anterior to brain ( Fig. 1D View Fig ). Plicated pharynx tubular in shape, one third of body length, located in anterior half of body ( Fig. 1B View Fig ). Oral pore situated at anterior end of pharynx, behind brain. Male gonopore, female gonopore, and sucker closely set on body center ( Fig. 1B View Fig ). Male copulatory apparatus consisting of large seminal vesicle, pair of prostatic vesicles, and armed penis papilla, located immediately posterior to pharynx ( Fig. 2A–D View Fig ). Spermiducal vesicles forming single row on each side of midline, separately entering into seminal vescle. Ejaculatory duct wide, with thick muscular layer, entering penis papilla. Prostatic ducts narrow, with muscular layer, attached to ejaculatory duct at proximal end of penis papilla. Pair of prostatic vesicles and seminal vesicle closely set to each other ( Fig. 2B View Fig ). Muscular sheath/bulb enclosing three vesicles not found ( Fig. 2B View Fig ). Pair of spherical prostatic vesicles coated with thin nonnucleated muscular wall, located on both sides of ejaculatory duct. Seminal vesicle oval, coated with thick muscular wall. Penis papilla armed with pointed tubular stylet, enclosed in penis pouch, protruding into male atrium ( Fig. 2C, D View Fig ). Penis sheath present between penis pouch and male antrum ( Fig. 2C, D View Fig ). Male atrium elongated anteriorly, lined with cilliated and muscularised epithelium ( Fig. 2A View Fig ). Female reproductive system immediately posterior to male reproductive system. Vagina short, leading from uterus to cement pouch ( Fig. 2A, D View Fig ). Cement glands numerous, concentrated around vagina and releasing their contents in cement pouch ( Fig. 2A View Fig ). Oviduct running on each side of main intestine, extending anteriorly and posteriorly to female copulatory apparatus; anterior and posterior branches of oviduct converging before joining proximal end of vagina ( Fig. 2A, D View Fig ). Lang’s vesicle absent.
Variation. Our specimens exhibited variation in the colour pattern on the dorsal surface. The body colour in general appearance ranged from orange to white depending on the gut contents. In addition, the density and distribution of the dark-brown pigments that form the mid-dorsal, meshlike band were different among the specimens examined; the transverse narrow dark-brown line in front of cerebral eyespots was interrupted in some specimens; another transverse white line in short distance behind dark-brown line was not clear in the largest specimen ( Fig. 3 View Fig ).
Diagnosis. Body elongated, usually rounded anteriorly; dorsal surface speckled with numerous orange maculae, blue dots, and dark-brown pigments, with dark-brown mesh-like band along median line; transverse dark-brown line running a short distance in front of similar, transverse white line on anterior part of body; a pair of free prostatic vesicles and a seminal vesicle located close together.
Etymology. The new specific name torquatum is a Latin adjective (- us, - a, - um), meaning “adorned with a neck chain or collar”. It was named after the anterior transverse white line, bending backward at the mid-point.
Distribution. Only known from the type locality, Shirahama, Wakayama, Japan.
Habitat. Intertidal, under rocks.
Sequences. Partial COI sequences (462 bp) from four individuals: LC429590 View Materials from the holotype ( ICHUM 5563 View Materials ), LC429589 View Materials and LC429591 View Materials from two paratypes ( ICHUM 5562 View Materials , ICHUM 5565 View Materials ), and LC429592 View Materials from a non-type specimen ( ICHUM 5567 View Materials ) . These four sequences were completely identical to each other.
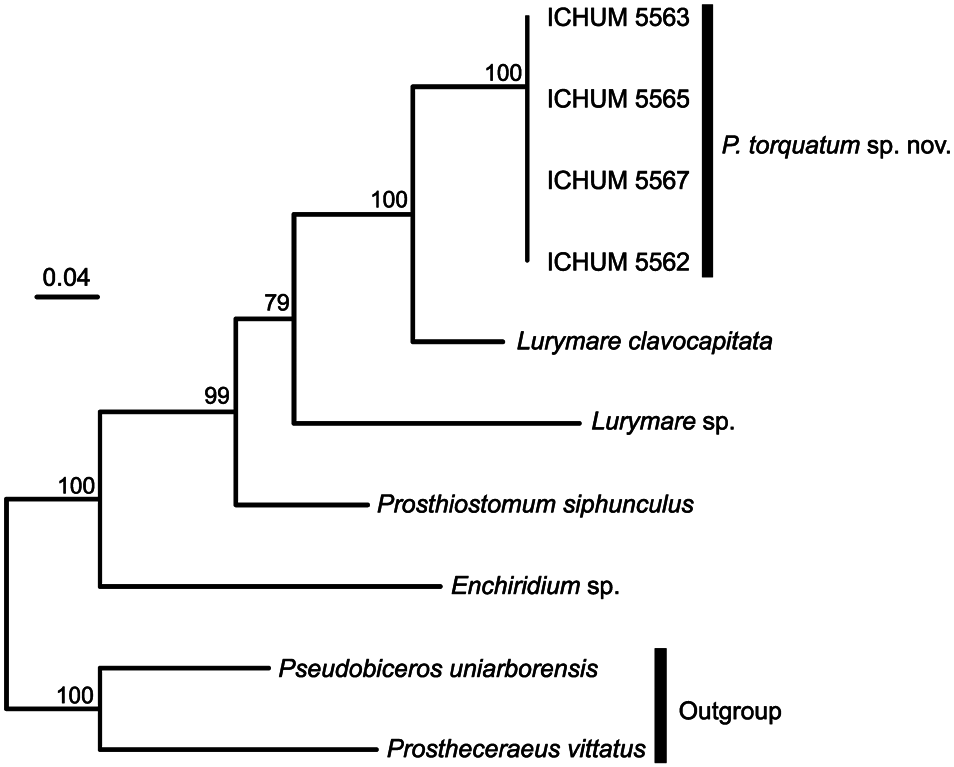
Phylogeny and genetic distance. In the phylogenetic tree, Prosthiostomum torquatum sp. nov. appeared as sister to Lurymare clavocapitata ; these two species differed by 0.094 uncorrected p -distance in terms of the 462-bp COI sequence. Prosthiostomum torquatum and P. siphunculus (Delle Chiaje, 1822) , the type species of Prosthiostomum , did not form a monophyletic group. Enchiridium sp. was sister to the rest of prosthiostomids ( Fig. 4 View Fig ).
Remarks. Our specimens belong to Prosthiostomum because they conform to Faubel’s (1984) generic diagnosis by having i) a pair of prostatic vesicles that are separated from each other, ii) a median frontal branch from the main intestine, and iii) a penis armed with a pointed tubular stylet. This definition applies to the type species, Planaria siphunculus Delle Chiaje, 1822 , and thus there is no serious taxonomic issue here. However, probably due to either misconception or oversight, Faubel (1984, pp. 232, 233) also included the three nominal species Prosthiostomum purum Kato, 1937 , Prosthiostomum cynarium Marcus, 1950 , and Prosthiostomum utarum Marcus, 1952 , which actually have a pair of prostatic vesicles enclosed together by a muscle bulb, and thus do not strictly fit the generic diagnosis. While the latter two have been transferred to Lurymare ( Bahia et al. 2014; Bahia and Schrödl 2018), P. purum is still left in the genus, awaiting further taxonomic scrutiny. As a result, there are 49 species currently included in Prosthiostomum . Of these, P. torquatum sp. nov. is similar to P. trilineatum Yeri and Kaburaki, 1920 and P. komaii Kato, 1944 in the dorsal colour pattern, which includes several transverse lines in the anterior part of the body and longitudinal stripes along the median line ( Yeri and Kaburaki 1920; Kato 1944). However, P. torquatum can be easily distinguished from both species by the orange maculae and blue dots scattered all over the dorsal surface. In actuality, we could have placed our new species in Lurymare due to its phylogenetic closeness to Lurymare clavocapitata , but we did not do so because our material lacked a common muscle bulb, a diagnostic character for Lurymare ( Faubel 1984) (see below).
Prosthiostomum torquatum and L. clavocapitata share a unique dorsal colour pattern among prosthiostomids: i) a translucent white body covered with numerous orange maculae and blue dots, ii) a transverse dark-brown line in front of the cerebral eyespots, directing backwards at the midpoint, and iii) another transverse white line running slightly behind the dark-brown one, likewise pointing posteriorly at the mid-point. Lurymare clavocapitata was established based on two specimens, the holotype and a paratype; while the white line was present in the holotype, it was absent in the paratype. The similarity between the two species is very likely due to their genetic/phylogenetic closeness ( Fig. 4 View Fig ).
Prosthiostomum torquatum differs from L. clavocapitata by the absence of the common muscle bulb, which encloses the whole male copulatory apparatus including the seminal vesicle, the two prostatic vesicles, the armed penis papilla, and the male atrium. We confirmed the difference by comparative examination on digital images of histological photomicrographs of the type specimens of L. clavocapitata . Prosthiostomum torquatum is also distinguishable from L. clavocapitata by their dorsal colour pattern; our new species has a mesh-like band along the mid-dorsal line formed by dark-brown pigments, although the density of the dark-brown pigments varies intraspecifically ( Fig. 3 View Fig ); L. clavocapitata has two discontinuous longitudinal lines composed of dark-brown pigments, instead of a mesh-like band. The COI p -distance between the two species, 0.094, is greater than the value of 0.045, which was observed between sympatric Notocomplana species morphologically distinguishable from each other ( Oya and Kajihara 2017), a value that is currently only available among the Polycladida , although these values may not be directly comparable as they belong to different suborders. While barcoding gaps in Prosthiostomidae are yet to be examined, we consider that P. torquatum and L. clavacapitata are likely to be two genetically independent entities.
The taxonomy of Lurymare in relation to Prosthiostomum requires revision because our phylogenetic tree based on COI indicates that these two genera as currently diagnosed are not monophyletic. The genus Lurymare was established by Du Bois-Reymond Marcus and Marcus (1968) for seven species, P. drygalskii Bock, 1931 , P. purum Kato, 1937 , P. delicatum Palombi, 1939 , P. russoi Palombi, 1939 , P. gabriellae Marcus, 1949 , P. matarazzoi Marcus, 1950 , and P. utarum Marcus, 1952 , on the basis of the presence of prostatic vesicles bound in a common muscle sheath which may include seminal vesicle. Later, Faubel (1984) gave a more strict definition to Lurymare so that it would contain members having a common muscle bulb that encloses the entire male copulatory apparatus including both of a pair of prostatic vesicles and a seminal vesicle. As a result, all species in Lurymare except for the type species, L. drygalskii (Bock, 1931) , were transferred to other genera, such as Prosthiostomum , Enchiridium , and Euprosthiostomum . At the same time, Faubel (1984) transferred another three species of Prosthiostomum , viz., P. monosorum (Schmarda, 1859) , P. singulare Laidlaw, 1904 , and P. katoi Poulter, 1975 , into Lurymare . In contrast, Prudhoe (1985) supported Du Bois-Reymond Marcus and Marcus’ (1968) taxon concept of Lurymare , retaining the original seven species in the genus, whereas Poulter (1975) considered that Lurymare should be a subgenus of Prosthiostomum . Bahia et al. (2014) redescribed P. utarum based on material collected in Praia das Conchas, about 250 km away from the type locality, Ilha de São Sebastião ( Brazil), and placed the species back in Lurymare , because the Praia-das-Conchas specimen possessed a muscle sheath, which was actually illustrated— but not mentioned—in Marcus’ (1952) original description, and was thus probably overlooked by Faubel (1984). Likewise, Bahia (2016) redescribed Euprosthiostomum matarazzoi as Lurymare matarazzoi . In addition, Bahia and Schrödl (2018) transferred P. cynarium Marcus, 1950 to Lurymare based on examination of the type material. A new species, L. clavocapitata , has been described by Marquina et al. (2015) from Lizard Island (Queensland, Australia) and placed in the genus based on Faubel’s (1984) strict definition, which currently amounts to eight species. According to Prudhoe (1989), the validity of Lurymare appears to be uncertain because a muscle sheath enclosing prostatic vesicles could develop as maturity increases in several Prosthiostomum species. Therefore, there is a possibility that the muscular developmental state observed in Lurymare represents a later ontogenetic stage of Prosthiostomum , and thus these two genera are actually synonymous. The results of our molecular phylogenetic analysis support this view. However, molecular information of the type species L. drygalskii is currently not available; synonymization of Lurymare with Prosthiostomum is thus premature. A taxonomic revision of Lurymare should involve a phylogenetic analysis of prosthiostomids with more gene markers and a dense taxon sampling including the type species of the constituent six genera, as well as careful examination of intra- and interspecific variation of various morphological characters that have been used to diagnose Lurymare .
| MT |
Mus. Tinro, Vladyvostok |
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |