Scaphander grandis ( Minichev, 1967 ), 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4646.2.12 |
|
publication LSID |
lsid:zoobank.org:pub:27EED11C-3EAF-4F13-B3BA-95AA53F9C342 |
|
persistent identifier |
https://treatment.plazi.org/id/03BC9604-FFAE-045B-57EE-63C4FF0D3D0E |
|
treatment provided by |
Plazi |
|
scientific name |
Scaphander grandis ( Minichev, 1967 ) |
| status |
comb. nov. |
Scaphander grandis ( Minichev, 1967) View in CoL comb. n.
( Figures 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Meloscaphander grandis Minichev, 1967: 130–134 View in CoL , figs. 25–29; 1969: 43.
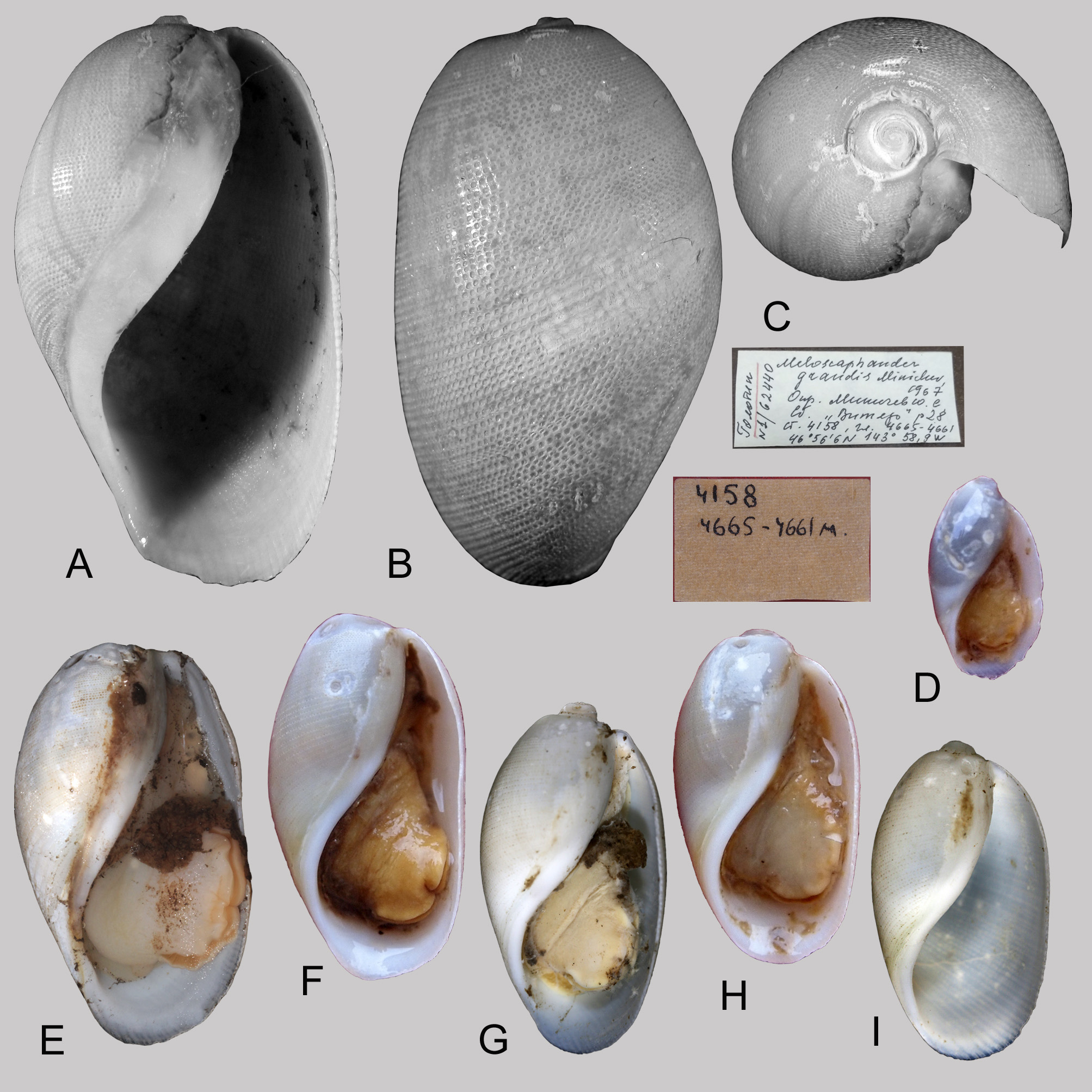
Type. Holotype, ( Figs. 3 View FIGURE 3 A–C), ZISP 1 View Materials /62440, North-East Pacific, 46º56.1’N, 143º58.9’W, 4,665 m depth, R/ V Vityaz, cruise 29, station 4158. Shell length 33 mm; shell width 18 mm (the shell and the soft body). GoogleMaps
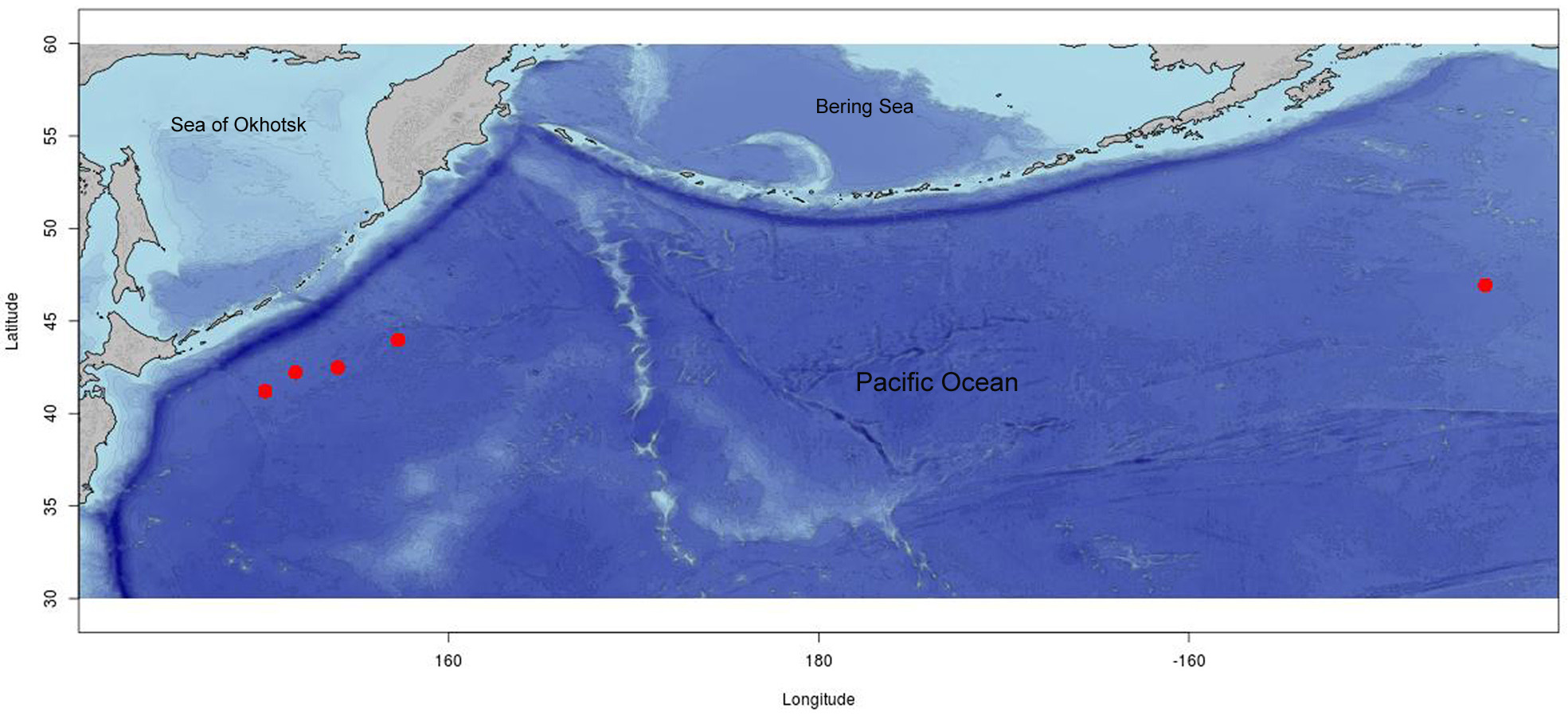
Other material examined. KuramBio I: one specimen (photo) from the Pacific abyssal plain adjacent to the Kuril-Kamchatka Trench, ZSM, station 1-13, 5,427 – 5,425 m depth; 3 specimens, MIMB 36540 View Materials , station 6-9, 5,293 – 5,307 m depth; 3 specimens GoogleMaps , ZISP 2 View Materials /62441, station 6-10, 5,295 –5,299 m depth GoogleMaps ; 3 shells, ZMFEFU XII 43753/Ga 9383, station 8-10, 5,124 –5,125 m depth; 1 specimen GoogleMaps , ZISP 3 View Materials /62442, station 10-10, 5,258 – 5,249 m depth GoogleMaps (for detailed collection information see Table 1 View TABLE 1 ).
Diagnosis. Shell large, ovoid, wider in 1/3–1/2 of length, with partly visible spire. Penial sac divided into anterior part with fold-like penis and posterior part with curved strand of vacuolated cells.
Description. Shell ( Fig. 3 View FIGURE 3 ). Large, ovoid, white, covered with pale yellow periostracum, with large body whorl and very small visible spire of 3–4.5 whorls. Studied specimens from 9.7 to 33 mm in shell height. Aperture as long as body whorl or slightly shorter, wider anteriorly and narrow posteriorly; outer lip thin, almost straight in the middle; inner lip S-curved, covered with thin callus extending to parietal wall. Shell sculpture consists of rounded or irregular pits ( Fig. 5D View FIGURE 5 ) in spiral lines with alternating rows of wider and narrow pits in smaller specimens. Animal can retreat into shell completely.
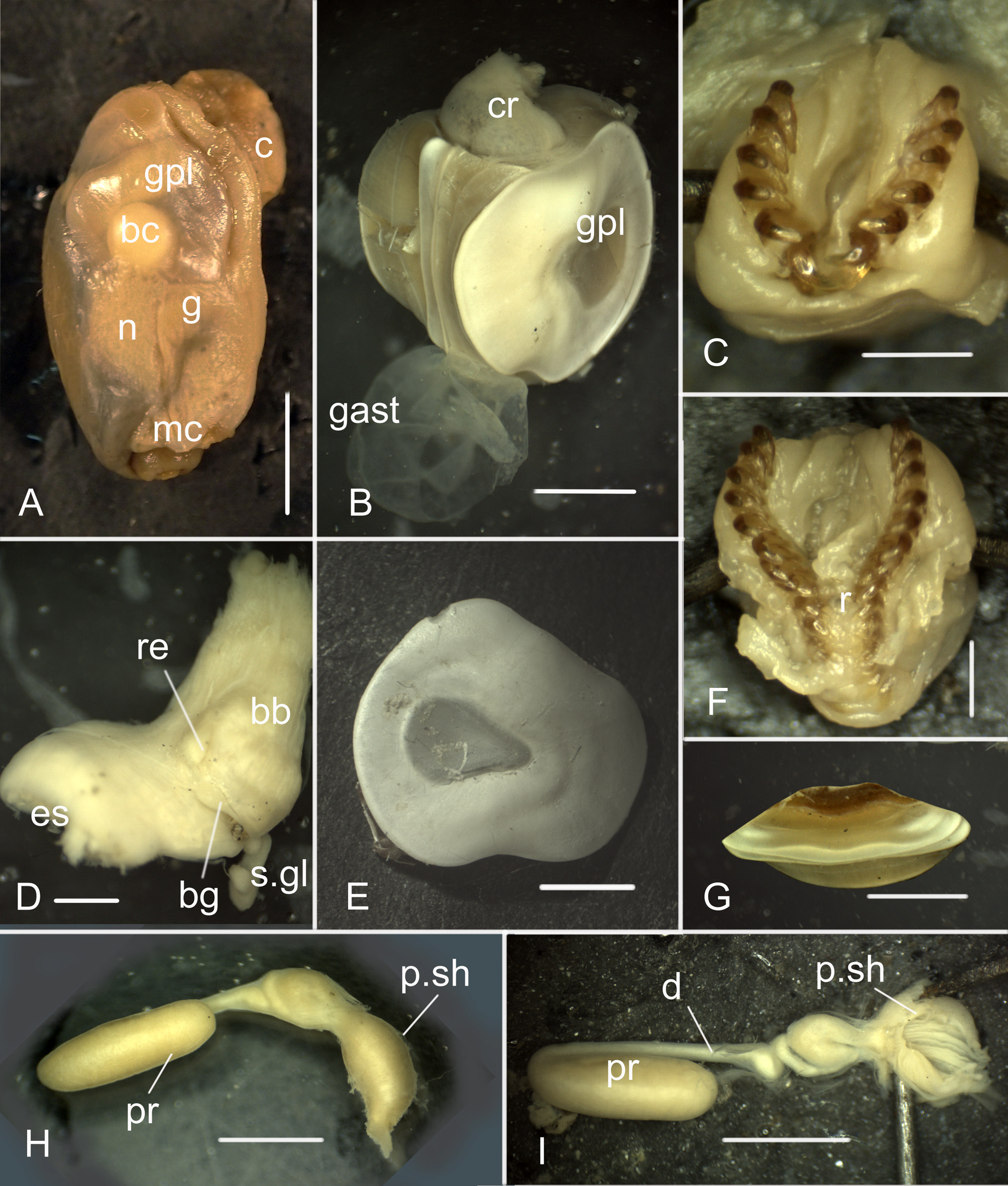
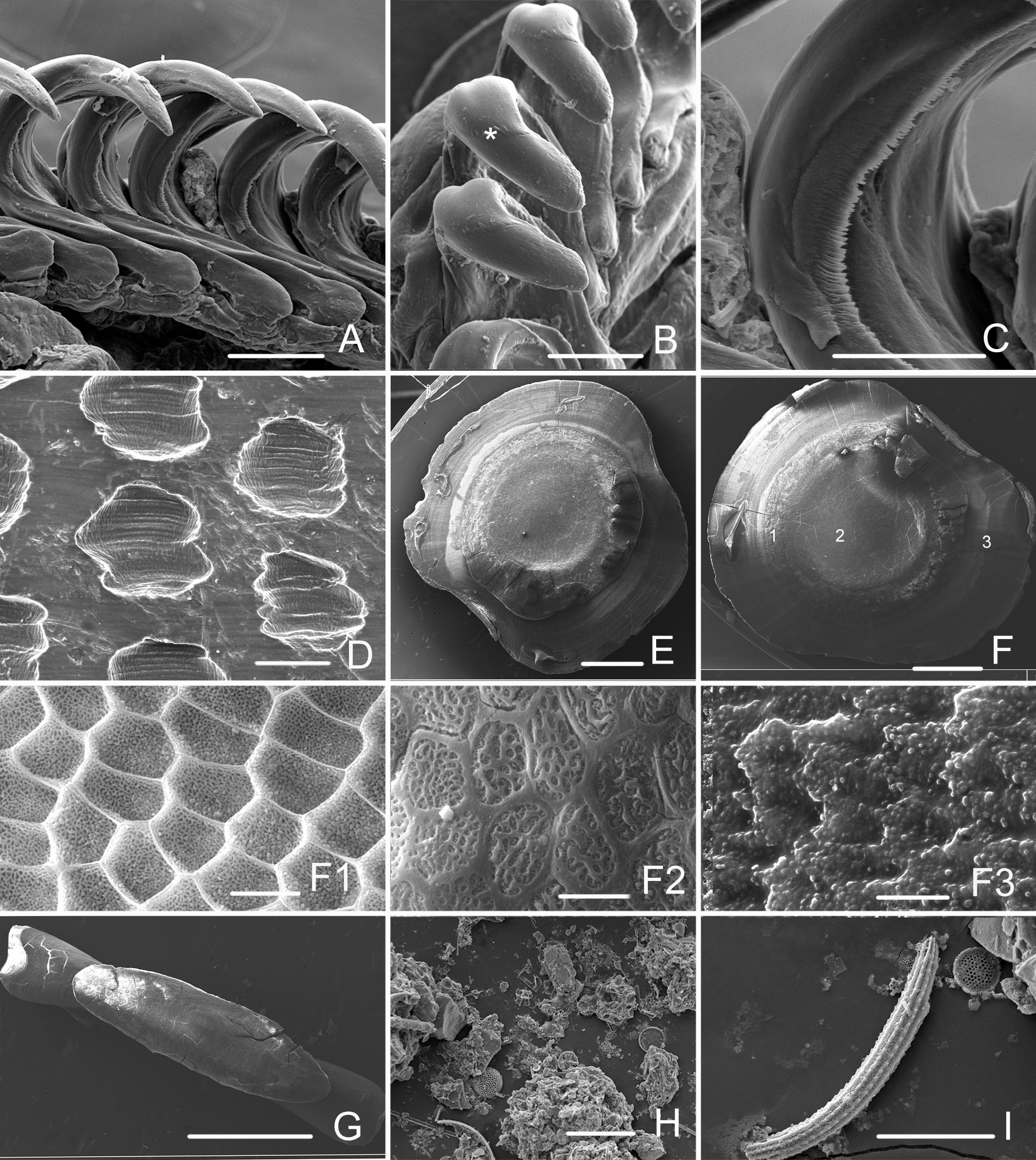
Radula. Formula 15×1.1(0).1; lateral teeth curved ( Figs. 5A, B View FIGURE 5 ), tapering at the top and bottom, with denticulation in the middle of the inner edge ( Figs. 5C View FIGURE 5 , 6A View FIGURE 6 ); they can diverge far from each other ( Fig. 4C View FIGURE 4 ) providing wide grip. Tips of lateral teeth covered with chitin crown ca. 100 µm in length ( Fig. 5B View FIGURE 5 ). Rachidian teeth rectangular (fig. 6A); vestigial, located in immature part of radular tape ( Fig. 4F View FIGURE 4 ), and in some posterior working rows of radula.
Digestive tract. Small buccal mass connects dorsally to short wide esophagus and short narrow twisted salivary glands ( Fig. 4D View FIGURE 4 ). Two strong muscle retractors attach to posterior end of buccal mass. Esophagus with thin-walled expansion (crop), opening into large muscular gizzard ( Fig. 4B View FIGURE 4 ). Gizzard with tree gizzard plates. Paired plates large, irregularly-oval (sub-quadrate) ( Figs. 4B, E View FIGURE 4 , 5E, F View FIGURE 5 ). Unpaired plate narrow, flattened laterally ( Figs. 4G View FIGURE 4 , 5G View FIGURE 5 ). All three plates with central thick area and thinner periphery. Microsculpture of periphery consists of small pits and tubercles ( Fig. 5F View FIGURE 5 3 View FIGURE 3 ); narrow area between central part and periphery consists of irregularly arranged polygons ( Fig. 5F View FIGURE 5 1 View FIGURE 1 ); polygons of central area filled with reticular matrix ( Figs. 5F View FIGURE 5 2 View FIGURE 2 ).
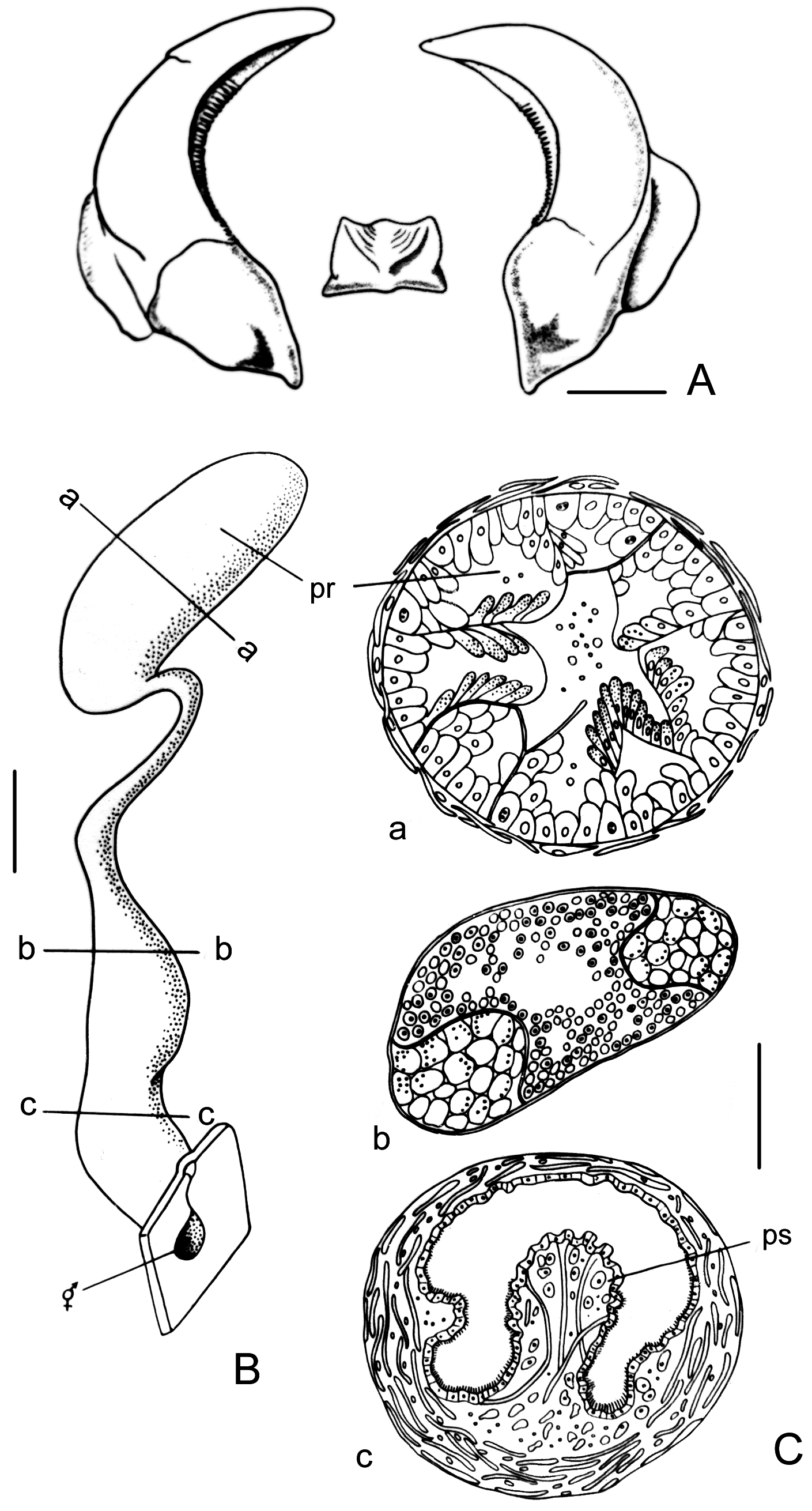
Male copulatory system. Prostate cylindrical ( Fig. 4H View FIGURE 4 ), rounded at the end. Prostate connected with penial sac by narrow duct folded near the sac. Penial sac ( Fig. 4I View FIGURE 4 ) elongate, divided into anterior part with fold-like penis and posterior part with curved strand of vacuolated cells (probably for supporting functions).
Additional data on copulatory system. The soft body of the holotype is too fragile to dissect the penial sac. The description of the male copulatory system in Scaphander grandis comb. n. was not published by Minichev (1967), but he described it in his PhD thesis ( Minichev 1965: 48–49, Fig. 33). To compare our specimens from the North-West Pacific with the type specimens, we provide here the drawing (see Fig. 6 View FIGURE 6 ) and the description of the copulatory system from Minichev’s thesis (1965, Fig. 33): “ Penial sac is lined by ciliary epithelium, … the penis proper is a muscular protrusion on the dorsal wall of the sac. The saccular part of prostate is partitioned by septa into numerous chambers; secretory epithelium is formed by large basophilic cells; their plasma is filled with gran- ules; nuclei are irregular in shape and contain two nucleoli. The duct of prostatic gland is filled with a mass of small-sized rounded nuclear elements (Fig. 33B-B). Strands of large vacuolated cells are arranged along the lateral walls of the duct. It is probable that these strands perform a supporting function; the nuclei are very small and ap- parently absent in some of cells ”.
Diet. Minichev (1967) reported that the crops of the studied specimens are filled with sand and debris of bi- valves. We found also foraminifers in the crop of the holotype. The fatty acid (FA) composition of S. grandis comb. n. was described by Kharlamenko et al. (2015) (based on the specimens (from station 6) which were nevertheless wrongly identified as Paracteocina sp.): according to these data, the FA composition of S. grandis is close to that of S. lignarius and may reflect a diet rich in microorganisms. The gizzard of the specimen from station 6-10 was filled with detritus, sand, and diatoms ( Figs. 5H, I View FIGURE 5 ).
Distribution. This species was originally described from the North-East Pacific abyssal plain at a depth of 4,665 m ( Minichev, 1967). In the present work, the geographic distribution record is expanded to the North-West Pacific abyssal plain, where the specimens were collected at a depth of 5,124 –5,427 m.
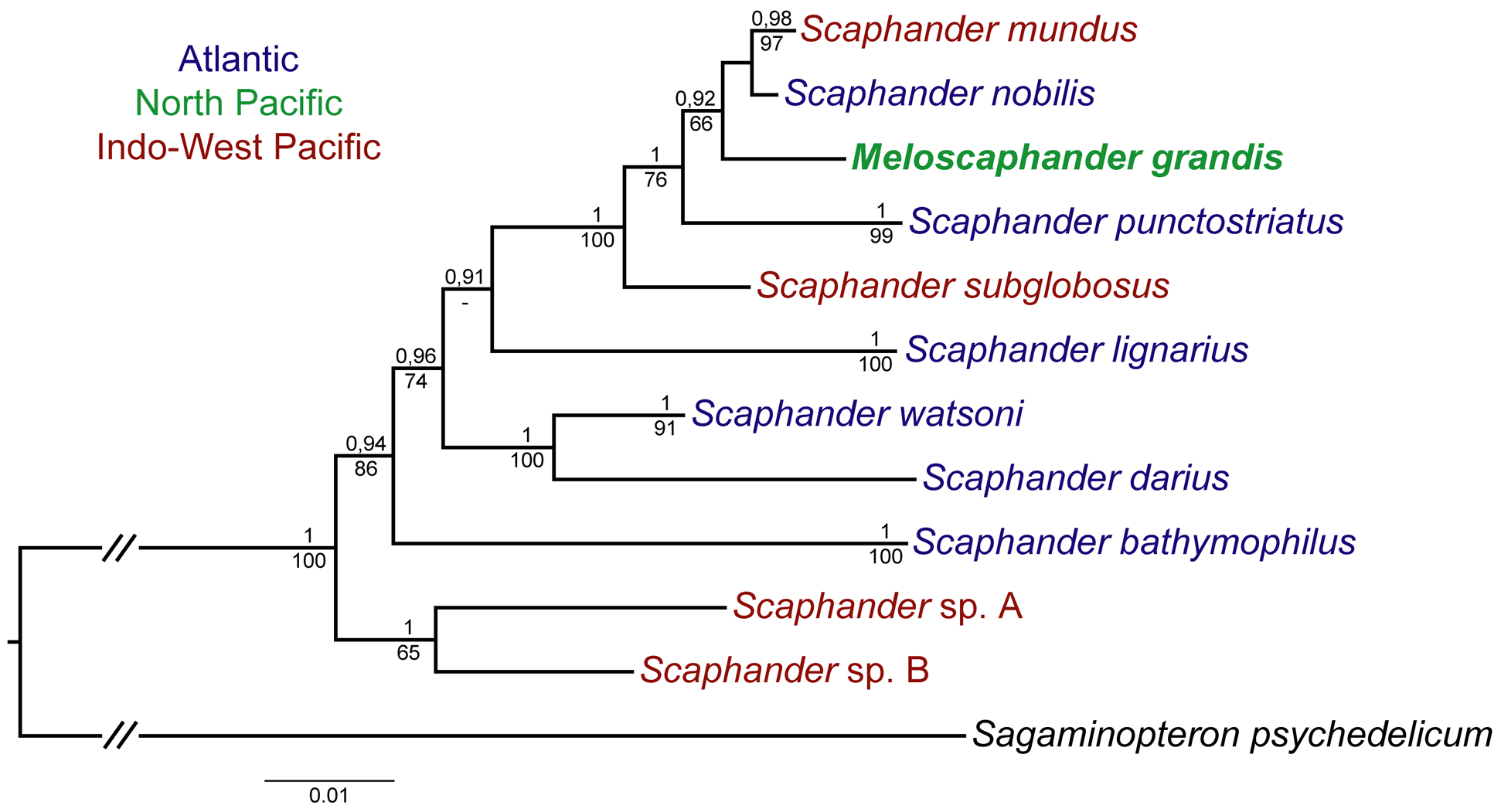
Remarks. We identified the studied specimens as Scaphander grandis comb. n. ( Minichev 1967) due to their similarity to the type specimens in shell, gizzard plates, and penial morphology (see Description). The morphologi- cal analyses confirm the placement of this species in the genus Scaphander : radula bears single, hamate lateral teeth and a vestigial rachidian tooth; penis is unarmed, connected to the prostate by a long duct; gizzard has two large sub-triangular plates and an unpaired one. All these traits are characteristics of the genus ( Eilertsen & Malaquias 2013a; Valdés & McLean 2015).
Scaphander grandis View in CoL comb. n. differs from the other two species, which were described as members of Meloscaphander View in CoL , in shell proportions and penial morphology ( Table 4 View TABLE 4 ). S. grandis View in CoL comb. n. differs from all the other species of Scaphander View in CoL by the visible shell spire. The sub-quadrate gizzard plates of S. grandis View in CoL comb. n. are similar to the plates of S. mundus Watson, 1883 View in CoL (see Valdés 2008, Fig. 42, E) and S. interruptus Dall, 1890 View in CoL (see Valdés & McLean 2015, Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ). The male copulatory system of S. grandis View in CoL comb. n. has the twisted narrow duct, as that in S. interruptus View in CoL (see Valdés & McLean 2015, Fig. 9), and the elongated penial sac divided into two parts, as in S. mundus View in CoL (see Valdés 2008, Fig. 40, B).
The shell outline of the smaller specimens of S. grandis View in CoL comb. n. ( Fig. 3D, G View FIGURE 3 ) slightly resembles Paracteocina vitjazi Minichev, 1966 View in CoL which may cause a confusion in identification ( Kharlamenko et al. 2015), but the latter species’ shell is narrower (see Kantor & Sysoev, 2006, Pl. 126, I), whorls have acute shoulder, and suture of the spire deeply canaliculated ( Minichev, 1966). Phylogenetic relationship of P. vitjazi View in CoL with other Scaphandridae View in CoL remains unclear.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Heterobranchia |
|
Order |
|
|
Family |
|
|
Genus |
Scaphander grandis ( Minichev, 1967 )
| Chaban, Elena M., Ekimova, Irina A., Schepetov, Dimitry M. & Chernyshev, Alexei V. 2019 |
Scaphander grandis
| Chaban & Ekimova & Schepetov & Chernyshev 2019 |
S. grandis
| Chaban & Ekimova & Schepetov & Chernyshev 2019 |
S. grandis
| Chaban & Ekimova & Schepetov & Chernyshev 2019 |
S. grandis
| Chaban & Ekimova & Schepetov & Chernyshev 2019 |
S. grandis
| Chaban & Ekimova & Schepetov & Chernyshev 2019 |
Meloscaphander grandis
| Minichev 1967: 130 |
Paracteocina vitjazi
| Minichev 1966 |
P. vitjazi
| Minichev 1966 |
Meloscaphander
| Schepman 1913 |
S. interruptus
| Dall 1890 |
S. interruptus
| Dall 1890 |
S. mundus
| Watson 1883 |
S. mundus
| Watson 1883 |
Scaphandridae
| G. O. Sars 1878 |
Scaphander
| Montfort 1810 |