Haematoloechus veracruzanus, León-Règagnon & Topan, 2018
|
publication ID |
https://doi.org/10.11646/zootaxa.4526.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:4DF63CE5-4838-46CA-BB0E-2F91841D5CB1 |
|
DOI |
https://doi.org/10.5281/zenodo.5970245 |
|
persistent identifier |
https://treatment.plazi.org/id/03B987D9-FFFC-881E-B6E0-00C7FD64FC4F |
|
treatment provided by |
Plazi |
|
scientific name |
Haematoloechus veracruzanus |
| status |
sp. nov. |
Haematoloechus veracruzanus n. sp.
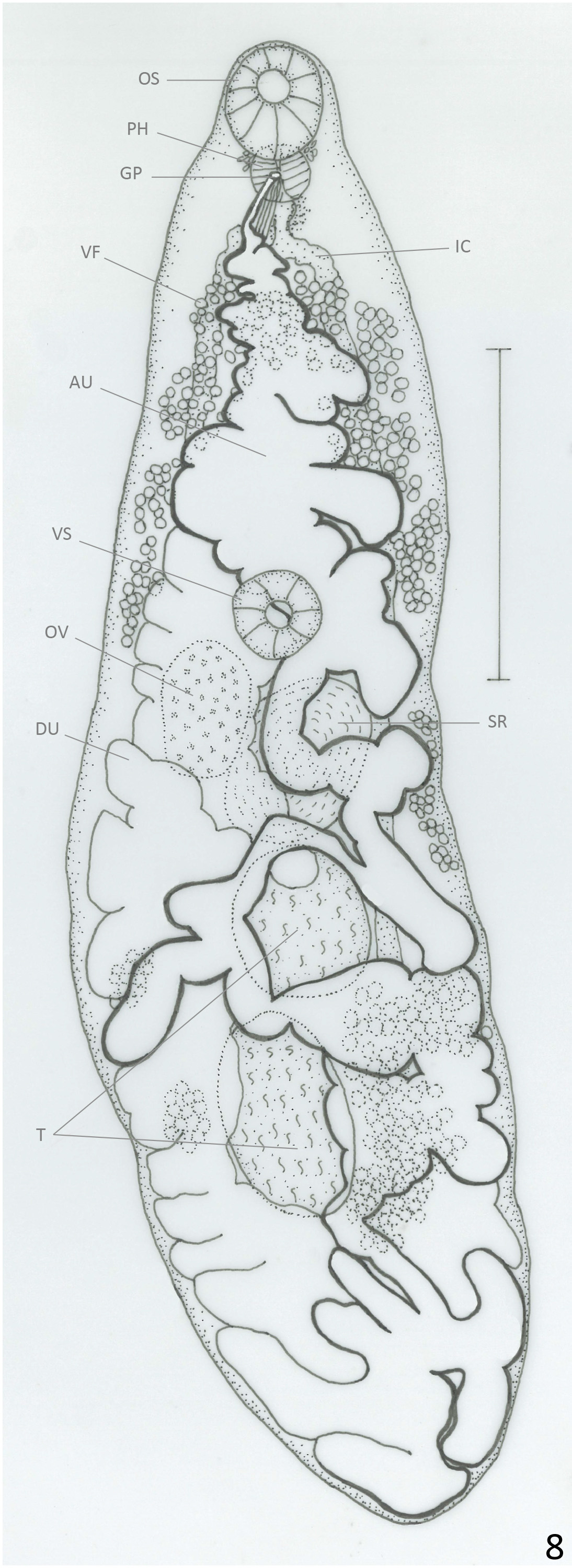
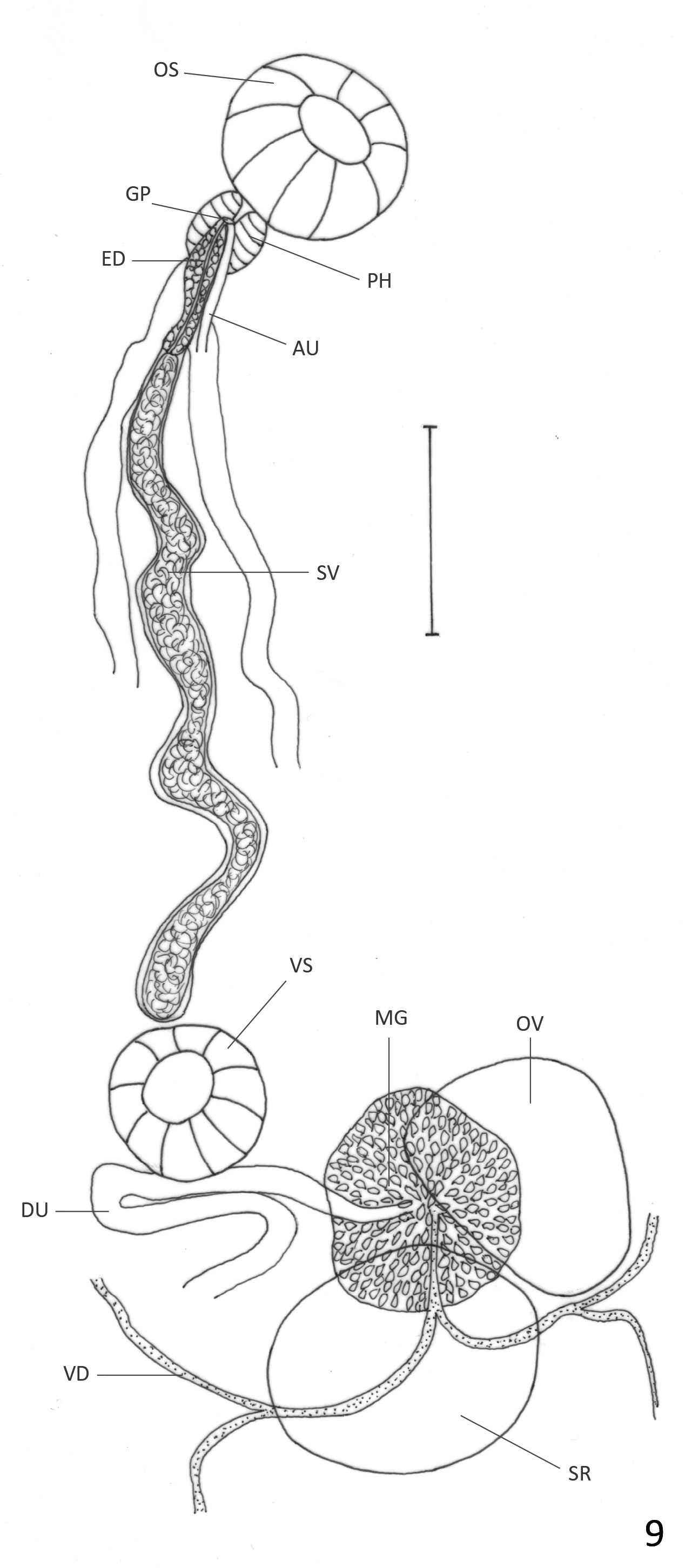
( Figs. 8 View FIGURE 8 & 9 View FIGURE 9 )
Type host: common marsh frog Rana vaillanti .
Type locality: Laguna Escondida, Los Tuxtlas, Veracruz, Mexico.
Site of infection: lungs
Holotype: CNHE 4087
Paratypes: CNHE 4086 , 4089 , 4090 , 10515 .
Other hosts and localities: common marsh frog R. vaillanti, Los Tuxtlas , Veracruz ( Paredes-Calderón et al. 2004 as H. complexus ); Catemaco, Veracruz (GenBank MG647801 View Materials , MG647802 View Materials , MG672439 View Materials ); Rio Grande leopard frog R. berlandieri , Laguna Higueras, Nuevo León ( León-Règagnon et al. 2005, as H. complexus ), Huauchinango, Puebla (León-Régagnon 2003, as H. complexus CNHE 10517), Rana sp., Tierra Quemada (CNHE 10518) and Rancho el Borbotón (CNHE 10519), San Luis Potosí, Mexico.
Etymology: Species name refers to Veracruz, the state in Mexico where the type locality is located.
Description: Based on 11 mature specimens. Body elongate, with slender anterior region; 3.5–5.2 (4.3) mm long, 0.80–1.64 (1.17) mm of maximum width at testicular region. Tegument aspinose, even in live specimens. Oral sucker subterminal, round, 203–365 (310) long, 252–409 (318) wide. Pharynx oval, 97–195 (154) long, 105– 170 (145) wide; oral sucker: pharynx ratio 1: 0.38–0.56 (0.48). Pharynx and anterior region of esophagus surrounded by abundant gland cells. Esophagus 65–122 (96) long, sometimes obscured by uterus. Ceca bifurcated at 381–649 (496) from anterior extremity. Ceca terminate blindly near posterior extremity. Ventral sucker round, 178–292 (245) long, 203–340 (265) wide, at 0.9–1.84 (1.4) mm (26%–38% (33%) of BL) from anterior extremity. Sucker length ratio 1: 0.64–0.94 (0.84). Testes 2, oval, oblique to almost tandem, inmmediately posterior to ovary. Anterior testis opposite to ovary, 308–771 (476) long, 292–674 (415) wide. Posterior testis 446–795 (604) long, 284–771 (433) wide. Cirrus sac reaches anterior border of ventral sucker, mostly obscured by ascending uterus; internal seminal vesicle, elongate, slightly coiled. Ejaculatory duct weakly muscular, 260–330 (295) long, surrounded by prostatic gland cells. Ovary oval, 349–576 (449) long, 227–608 (335) wide; at 0.92–2.1 (1.6) mm (26%–42% (37%) of BL) from anterior extremity. Seminal receptacle adjacent and partially overlaps ovary; 349– 771 (548) long, 341–649 (429) wide. Mehlis gland dorsal to seminal receptacle. Laurer’s canal not observed. Vitellaria in clusters overlapped with each other, distributed laterally, dorsally invade space between ceca in their anterior limit and in post-testicular region. Anterior limit of distribution 471–1000 (662) (10%–23% (15%) of BL) from anterior end. Follicles extend asymmetrically, to level of posterior testis on ovarian side of body, and halfway between posterior testis and posterior end on side opposite to ovary. Uterine loops fill intra- and extracecal space, partially overlapped with testes and ovary. Descending part of uterus form several diagonal loops that frequently bend anteriorly or posteriorly and form short longitudinal extracecal loops. Uterus forms two diagonal uterine loops oriented anteriorly on each side of posterior end of body, sometimes reaches halfway between posterior end and posterior testis. Ascending part of uterus form transverse or diagonal loops on side opposite to ovary, they frequently invade ovarian side and bend anteriorly or posteriorly to form short longitudinal extracecal loops. Distal uterus fills entire preovarian region with diagonal loops. Genital pore median, ventral to anterior region of pharynx. Eggs dark brown, 34–40 (37) long, 18–23 (20) wide. Excretory vesicle not observed. Excretory pore terminal.
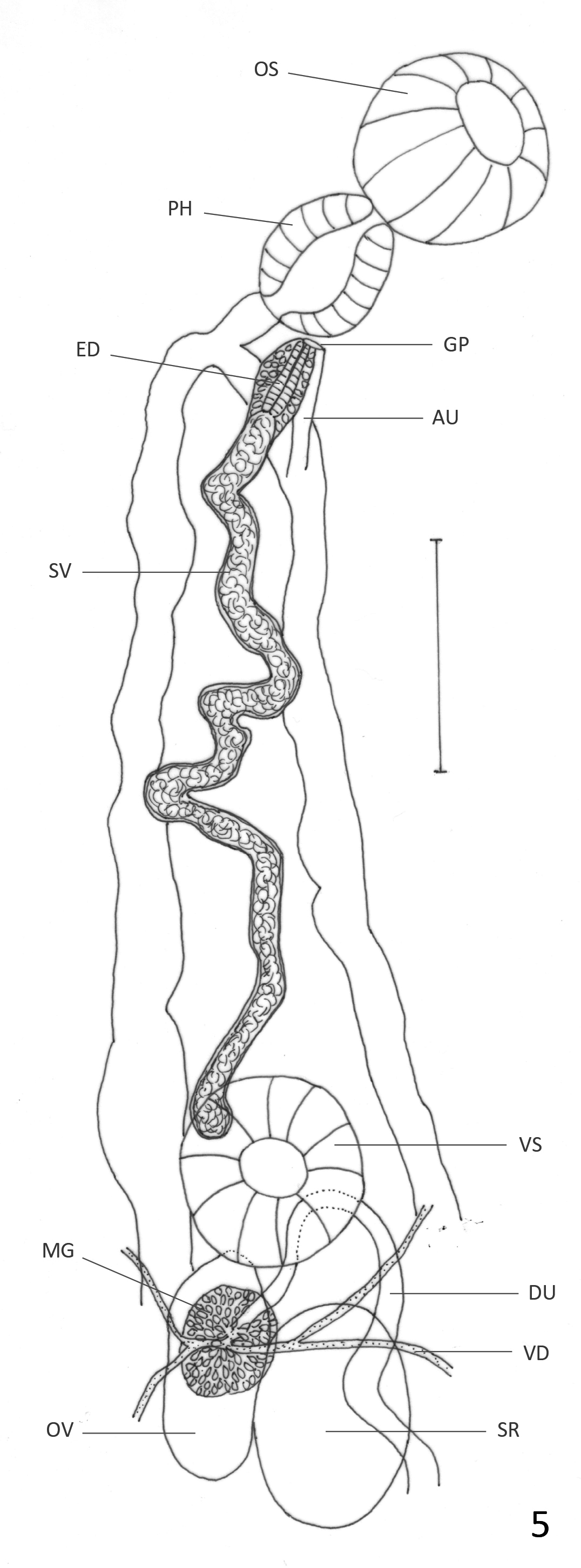
Remarks: Haematoloechus veracruzanus n. sp. resembles H. arequipensis , H. caballeroi , H. complexus , H. danbrooksi , H. dollfusinum , H. elongatus , H. fuelleborni , H. humboldtensis , H. illimis , H. kernensis , H. longicollum , H. medioplexus , H. meridionalis , H. occidentalis n. sp., H. parcivitellarius , H. pukinensis , and H. pulcher and differs from other American species in the genus by having uterine loops invading the extracecal area, and by lacking longitudinal uterine loops reaching at least the level of the posterior testis. It differs from H. danbrooksi , H. medioplexus , and H. meridionalis in the size of the ventral sucker compared to the oral sucker, which is less than one third in those four species vs more than half in H. veracruzanus n. sp. ( Table 2) ( Stafford 1902; León-Règagnon et al. 2001; León-Règagnon & Paredes-Calderón 2002). Haematoloechus veracruzanus n. sp. differs from H. arequipensis in having oval rather than lobed testes ( Ibañez & Córdoba 1979), and differs from H. illimis and H. dollfusinum in the shape of the ovary, which is lobed in those species ( Caballero 1942a) and oval in H. veracruzanus . The presence of diagonal uterine loops directed anteriorly at the posterior end of the body differentiates H. veracruzanus n. sp. from H. humboldtensis , H. longicollum , H. parcivitellarius , and H. pulcher , in which the diagonal uterine loops are either absent or directed posteriorly ( Caballero 1942b; Bravo–Hollis 1943; Zamparo et al. 2011; León-Règagnon & Romero–Mayén 2017). Haematoloechus veracruzanus n. sp. differs from H. fuelleborni , in having a larger ventral sucker compared to the oral sucker (1:0.5 vs 1: 0.84 in H. veracruzanus n. sp.), and in the distribution of the vitellaria; while they are limited to two groups, one anterior to the ventral sucker and other posterior to the testes in the South American species, they are distributed continuously extending asymmetrically, from the region anterior to the ventral sucker to the level of the posterior testis on the ovarian side of the body, and halfway between the posterior testis and the posterior end on the side opposite to the ovary in H. veracruzanus n. sp. ( Travassos & Darriba 1930). It differs from H. complexus and H. elongatus in the arrangement of the uterine loops. While in H. complexus and H. elongatus the uterine loops are transverse in the post-acetabular region ( Caballero & Sokoloff 1934; Bolek & Janovy 2007a), in H. veracruzanus n. sp. the uterine loops are transverse or diagonal often bending to form short longitudinal loops that can be oriented anteriorly or posteriorly. Haematoloechus elongatus is also much larger in body size than H. veracruzanus n. sp. ( 9.5 mm vs 4.3 mm). Haematoloechus veracruzanus n. sp. differs from H. occidentalis n. sp. in the arrangement of the uterine loops; while the descending and ascending loops form two lateral fields in the post-testicular region in H. occidentalis n. sp., the ascending uterine loops frequently invade both sides of the body in H. veracruzanus n. sp. ( Figs. 6 View FIGURE 6 & 8 View FIGURE 8 ). Haematoloechus veracruzanus n. sp. differs from H. kernensis in the arrangement of the uterus, which forms a few diagonal loops in the pre-acetabular and post-testicular region in that species, while fills with transverse or diagonal loops in both areas in H. veracruzanus n. sp., and in the distribution of the vitellaria, which do not invade the intercecal region in the post-testicular area in H. kernensis ( Ingles 1932) . The new species also differs from H. pukinensis in the arrangement of the uterine loops; while in H. pukinensis the ascending part of the uterus forms a few transverse loops in the pre-acetabular region ( Ibáñez & Córdoba 1979), in H. veracruzanus n. sp. the ascending uterus entirely fills the pre-acetabular region. Haematoloechus veracruzanus n. sp. most closely resembles H. caballeroi , but the two species can be differentiated by the arrangement of the uterus. While in H. caballeroi , the descending and ascending uterus form transverse loops that sometimes become diagonal and are oriented anteriorly in the extracecal region, in H. veracruzanus n. sp. the uterine loops are transverse or diagonal, frequently bending to form short longitudinal loops in the extracecal region that can be oriented anteriorly or posteriorly; also, the ejaculatory duct in H. caballeroi is strongly muscular while it is weakly muscular in H. veracruzanus n. sp. ( Figs. 5 View FIGURE 5 & 9 View FIGURE 9 ).
Haematoloechus veracruzanus n. sp. is another species that was differentiated using DNA sequences (León- Règagnon & Brooks 2003, Genbank AF531857 View Materials ; León-Règagnon 2010, GenBank HQ141684 View Materials (Ha15), HQ141701 View Materials (Ha51)) and is included in the "complexus group".
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
SuperFamily |
Plagiorchioidea |
|
Family |
|
|
Genus |
Haematoloechus veracruzanus
| León-Règagnon, Virginia & Topan, Janet 2018 |
Haematoloechus veracruzanus
| León-Règagnon & Topan 2018 |