Jassa marmorata Holmes, 1905
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4939.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:F33F42D0-A139-4CE3-97D7-1314C12CF86B |
|
DOI |
https://doi.org/10.5281/zenodo.4580558 |
|
persistent identifier |
https://treatment.plazi.org/id/03B487DA-FF98-D92C-C9C8-1B86FC74F8AE |
|
treatment provided by |
Plazi |
|
scientific name |
Jassa marmorata Holmes, 1905 |
| status |
|
Jassa marmorata Holmes, 1905 View in CoL
( Table 10 View TABLE 10 , Figs 15–21 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 View FIGURE 20 View FIGURE 21 )
Synonyms: see Conlan (1990).
Diagnosis.
Both sexes:
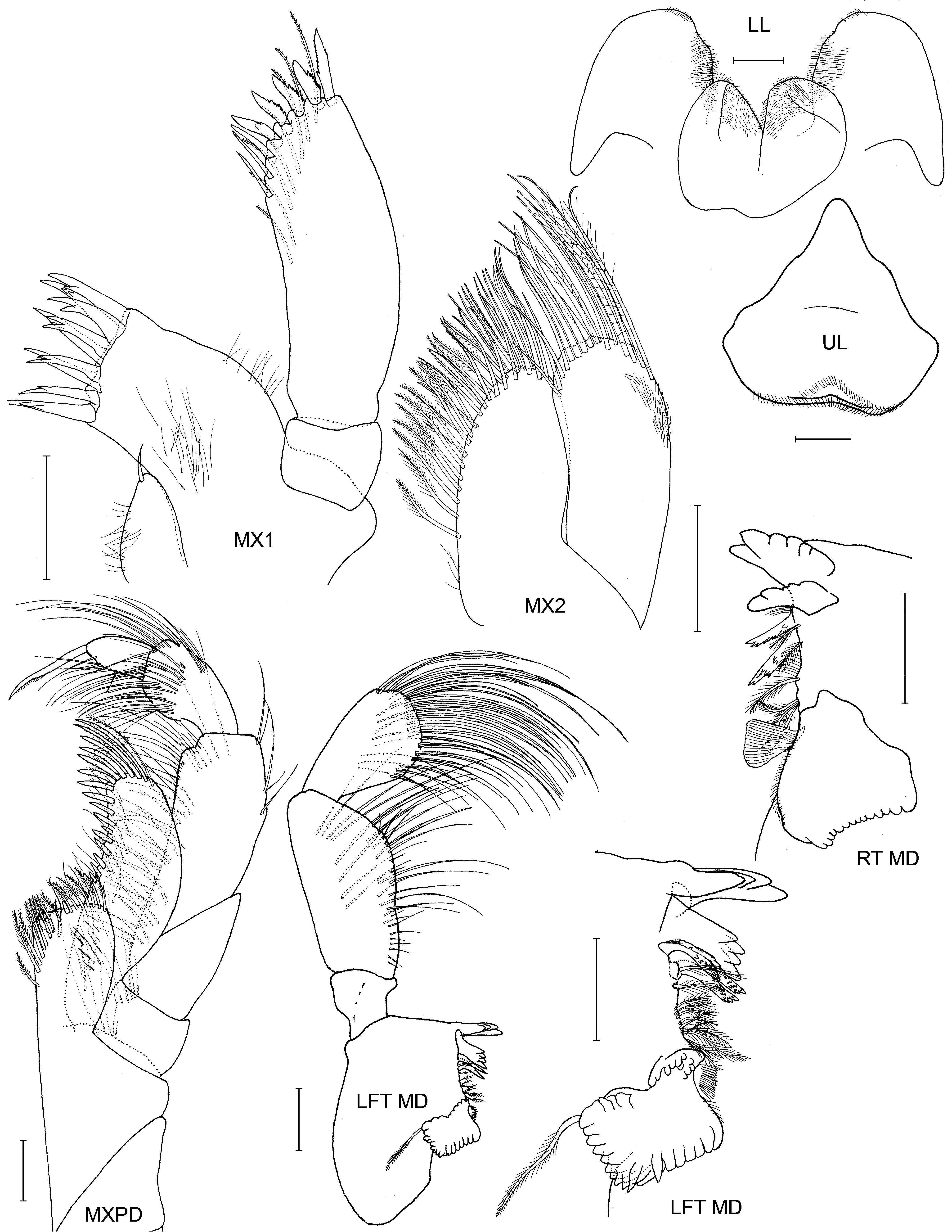
Mandibular palp: article 2, dorsal margin without a fringe of setae.
Maxilla 1: without a seta or setal cluster at the base of the palp article 1.
Gnathopod 1: basis, anterolateral margin with a few short setae along its length; carpus with a (usually) single or a small cluster of short setae at the anterodistal junction of the propodus (setae <25% of anterior margin length andslightly lateral).
Gnathopod 2: basis witha row of closely spaced setae along the anterolateral margin (at least some setal lengths>40% of the basis width); carpus and propodus, setae on the anterior margin short and simple (setal length <basis width).
Pereopods 5–7: propodus not expanded anteriorly.
Uropod 1: ventral peduncular spinous process underlying about 1/3 of the longest ramus.
Uropod 3: inner ramus without spines mid-dorsally (with only the single apical spine).
Telson: tip without apical setae (only the usual short setae at each dorsolateral cusp).
Thumbed male:
Antenna 2: large individuals with plumose setae on the flagellum and peduncular article 5.
Gnathopod 2: propodus, palmar defining spines not produced on a ledge, present in small thumbed males but absent in large thumbed males. In minor males, the thumb is distally acute, short relative to body length and located on the distal half of the propodus. The dactyl is not centrally toothed. In major males, the thumb is distally squared, longer relative to body length and on the proximal half of the propodus. The dactyl is expanded close to the junction with the propodus but is not centrally toothed.
Adult female:
Antenna 2: large animals with plumose setae on the flagellum and peduncular article 5.
Gnathopod 2: propodus, palm concave, palmar defining angle acute.
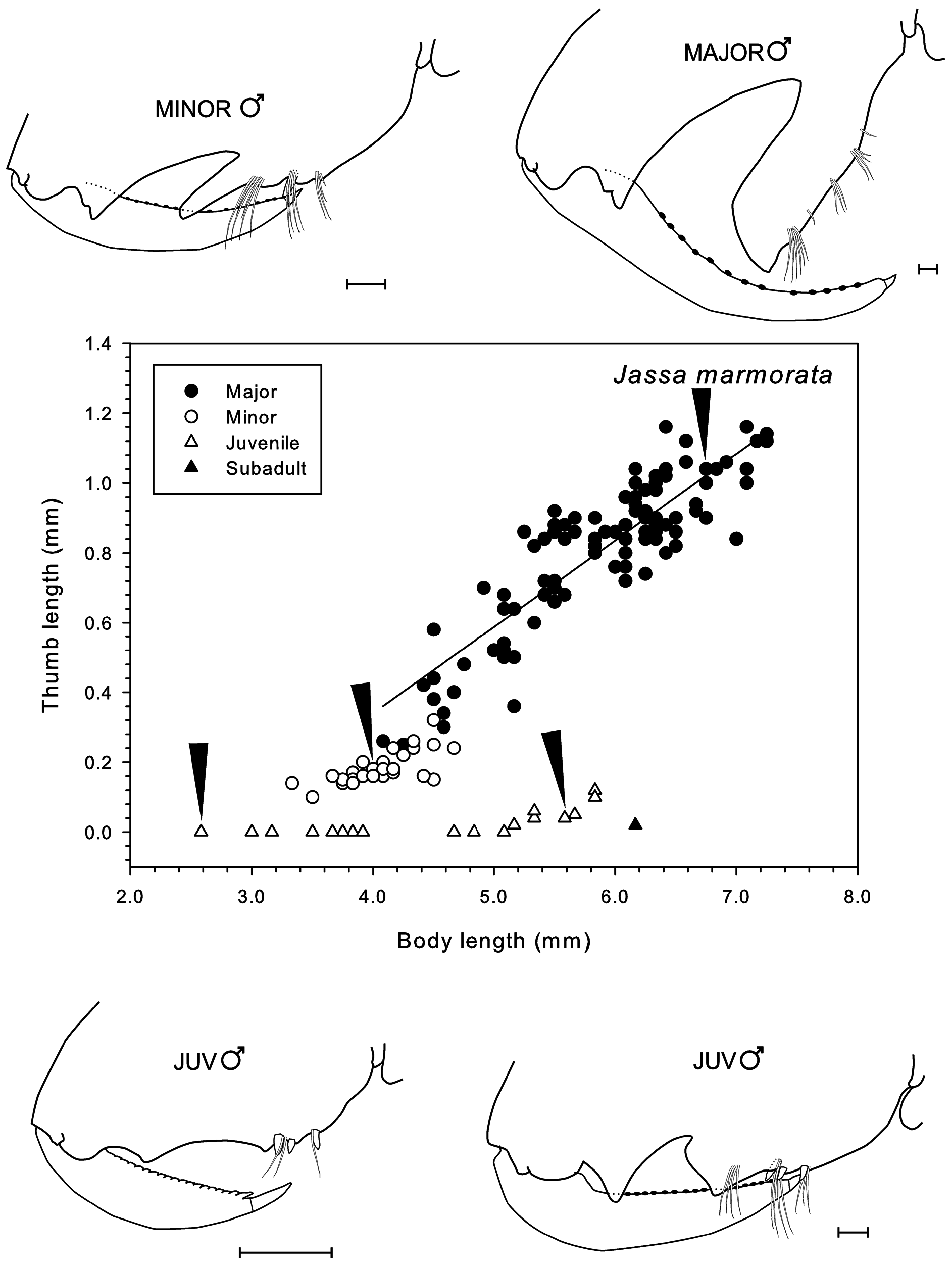
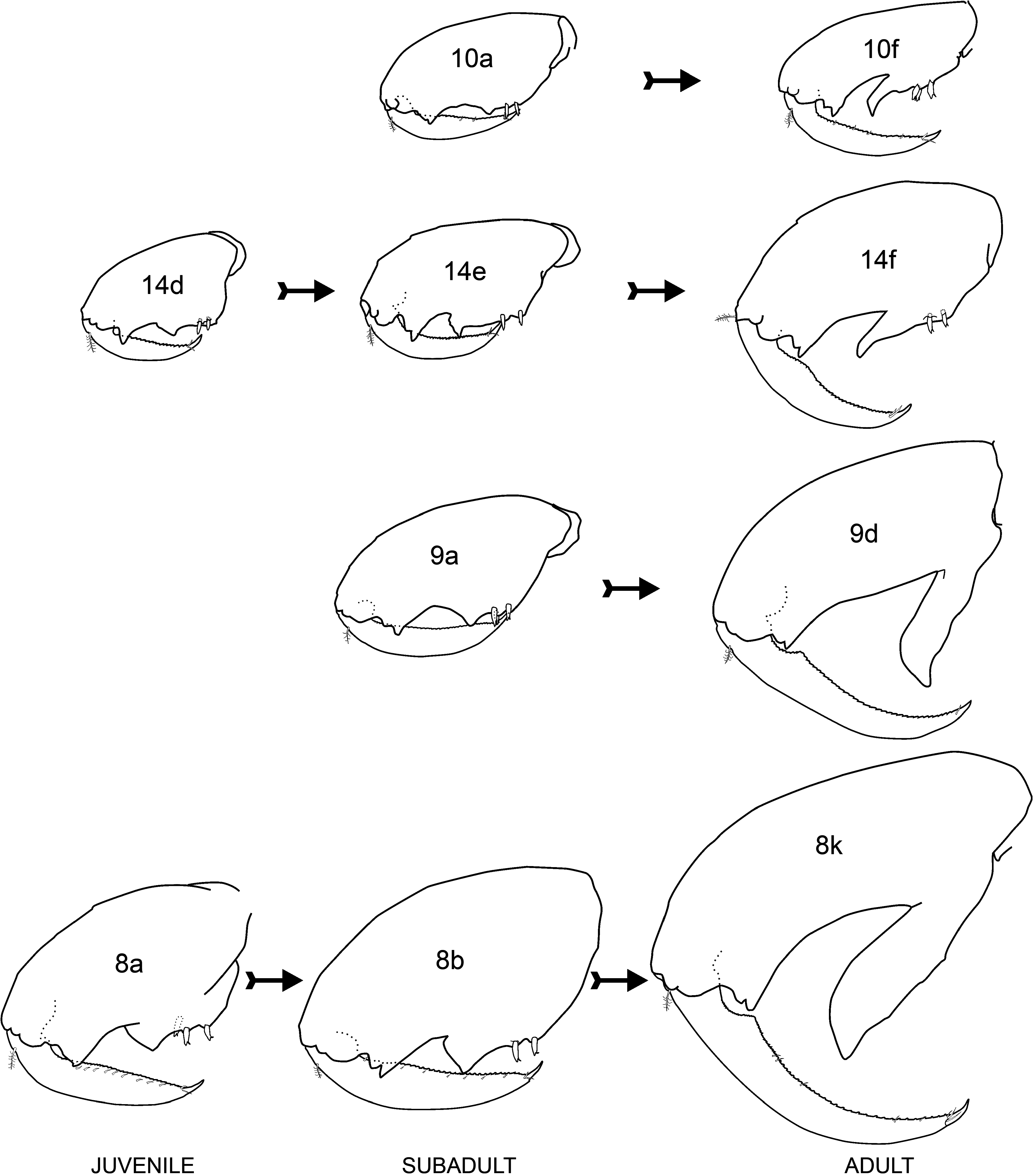
Remarks. Jassa marmorata has often been mis-identified as J. falcata in the past ( Conlan 1990). Sexton and Reid (1951) studied the development of J. falcata and their J. falcata “broad form” is in fact J. marmorata . The transformation of four males from non-thumbed to thumbed state in aquarium cultured specimens is reproduced in Fig. 18 View FIGURE 18 from Sexton and Reid’s (1951) plates. This shows that some subadult males have a small “pre-thumb” in the palm of gnathopod 2 ( Figs 8a, b View FIGURE 8 , 14 d, e View FIGURE 14 and 18 View FIGURE 18 ) while others do not ( Figs 9a View FIGURE 9 , 10a View FIGURE 10 and 18 View FIGURE 18 ). The appearance of a pre-thumb appears to occur only in larger juveniles ( Fig. 16 View FIGURE 16 ). Fig. 18 View FIGURE 18 also shows that while the palmar defining spines are retained in small thumbed males, they are lost in large thumbed males.
Sexton and Reid (1951) produced intersexes with a short, narrow thumb and small, setose brood plates by inbreeding siblings generated by a pair collected in the wild. Although the intersexes mated with female siblings, the offspring did not survive to maturity. Two intersexes and an ovigerous female were also found in NMNH 148787, 3–38, taken in a dredge at 18–27 m over sandy, weedy bottom inside the northern point of entrance to Magdalena Bay, Lower California, on July 18, 1938. Both intersexes had all the distinguishing features of J. marmorata but short thumbs on the second gnathopods and a toothed dactyl similar to that in minor forms of J. falcata and J. staudei .
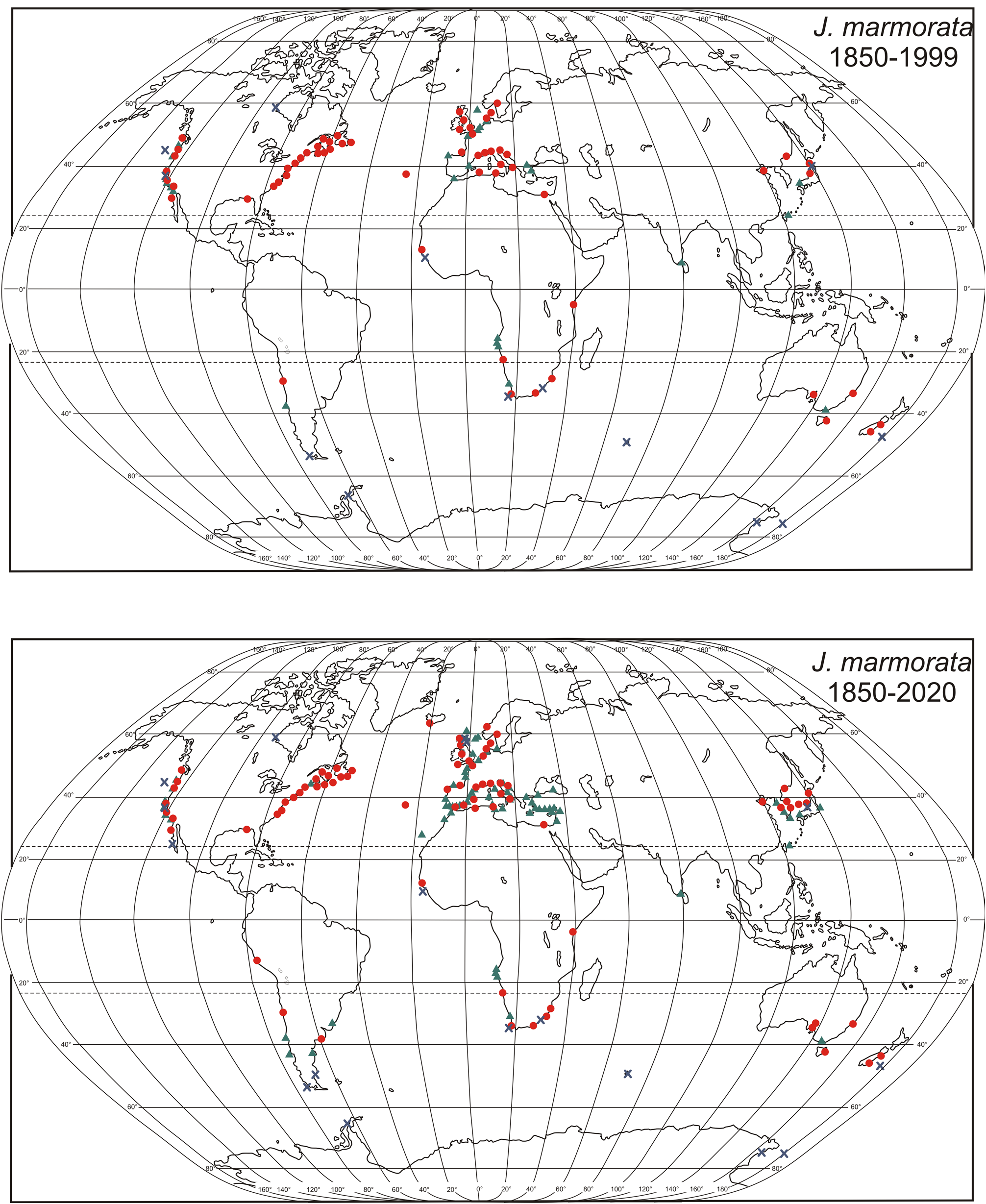
Jassa marmorata is morphologically similar to J. valida and the two overlap in distribution at the junction of their ranges in North Carolina, which is a biogeographic break for many species ( Pappalardo et al. 2015). Distributions listed in OBIS for J. marmorata for the coasts of the US south of North Carolina, including the Gulf of Mexico require confirmation because they may be for mis-identified J. valida . Asimilar southerly range into the Gulf of Mexico and on the coasts of Cuba and the Bahamas listed by Conlan (1990) may also be for J. valida , not J. marmorata . It is possible that some or all records of J. marmorata on the coasts of Uruguay and Argentina described by Alonso de Pina (2005) are unrecognized J. valida , since J. valida is already known from this coast. The most dependable character to distinguish the two species of any size is the lack of apical setae on the telson of J. marmorata and presence in J. valida . However, viewing this character requires moving the third uropods out of the way of the telson (this can be done by holding the animal on its side and bending the uropods downwards with a fine needle). It also requires that the usual pair of setae at the the telson knobs, which project dorso-distally, are not confused with the apical setae, which project distally. The other diagnostic characters of J. marmorata , which are easier to view, can be used for adult and subadult specimens.
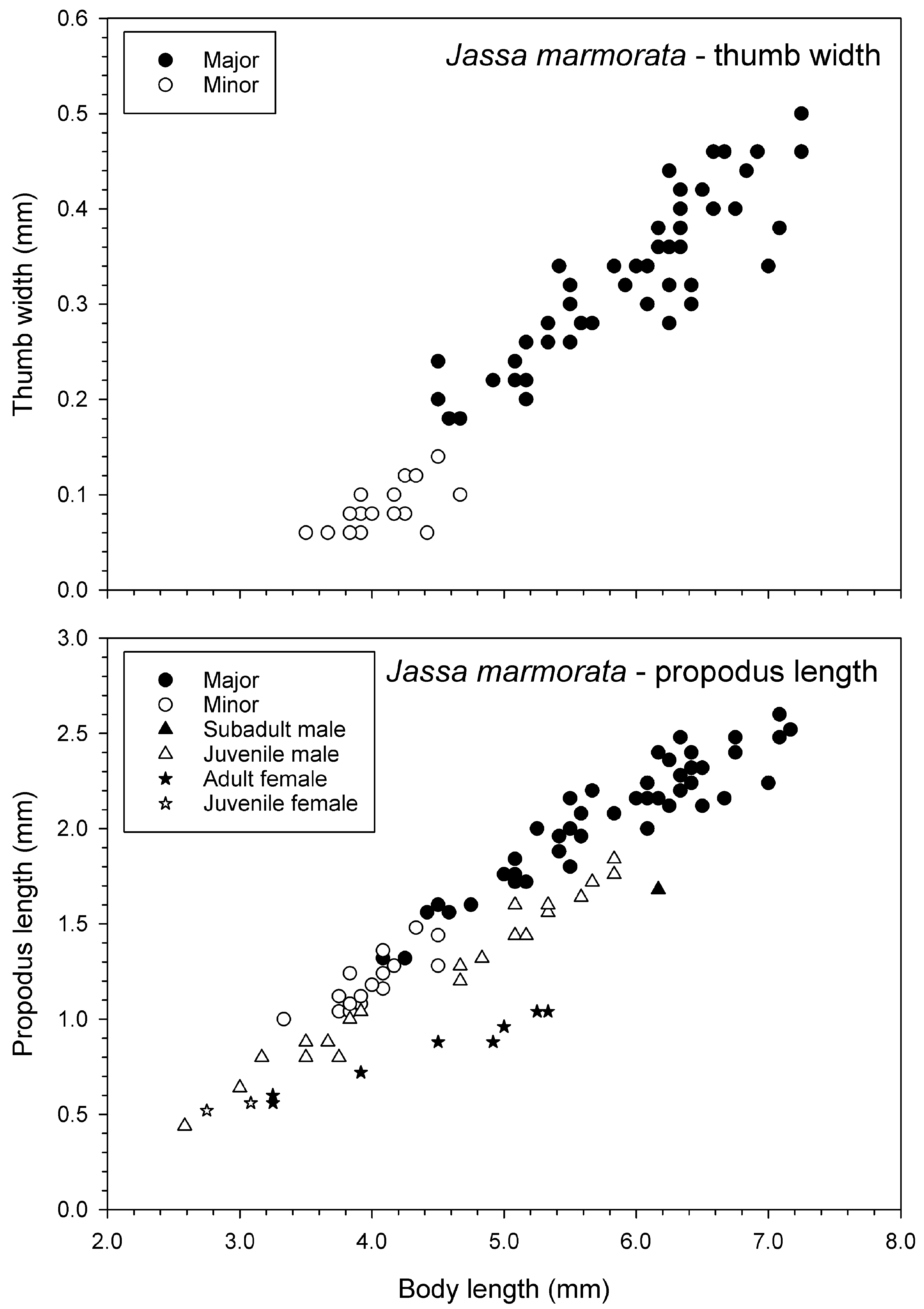
Due to the availability of a large number of specimens from the neotype population (162 adult and juvenile males measured for Fig. 16 View FIGURE 16 ), there were 10 major form and 17 minor form males that overlapped in body length (4.08–4.67 mm) for comparison of thumb length relative to body length. A Kruskal-Wallis test indicated that thumb lengthwassignificantly longerinthe major thanminorforms of thissizerange (HKW = 4.017, p <0.001; median thumb length 0.39 vs 0.22 mm, respectively). The thumbs also appeared wider in major than minor forms ( Fig. 17 View FIGURE 17 ) although there were insufficient specimens with a common range of body length to test this. The second gnathopod propodus length was significantly greater in major form adult males (mean 1.812 ± 0.245 mm) than juvenile males (1.533 ± 0.200 mm) of the same body length range (4.08–5.83 mm) (t -test, t = 3.458, p = 0.001, n = 27 major forms and 12 juveniles) ( Fig. 17 View FIGURE 17 ). Similarly, the juvenile males had a significantly longer propodus than the adult females of thesamebodylengthrange (3.25–5.33 mm) (HKW = 2.195, p <0.05, median 1.20 mmforjuvenile males, n = 15 and 0.88 mm for adult females, n = 8) ( Fig. 17 View FIGURE 17 ).
The SEM image of the telson ( Fig. 20 View FIGURE 20 ) shows paired short plumose setae to either side of the telson nubs and associated long, erect seta dorsal to each nub. The nub magnifications show that the nubs are actually cusped and the erect setae are ringed at their tips by rows of scales. Presumably, the erect setae have a sensory function, perhaps for position within the tube and the cusped nubs may assist with gaining a purchase within the tube. Possibly the plumose setae are mechanoreceptive as well (Kauffmann 1994).
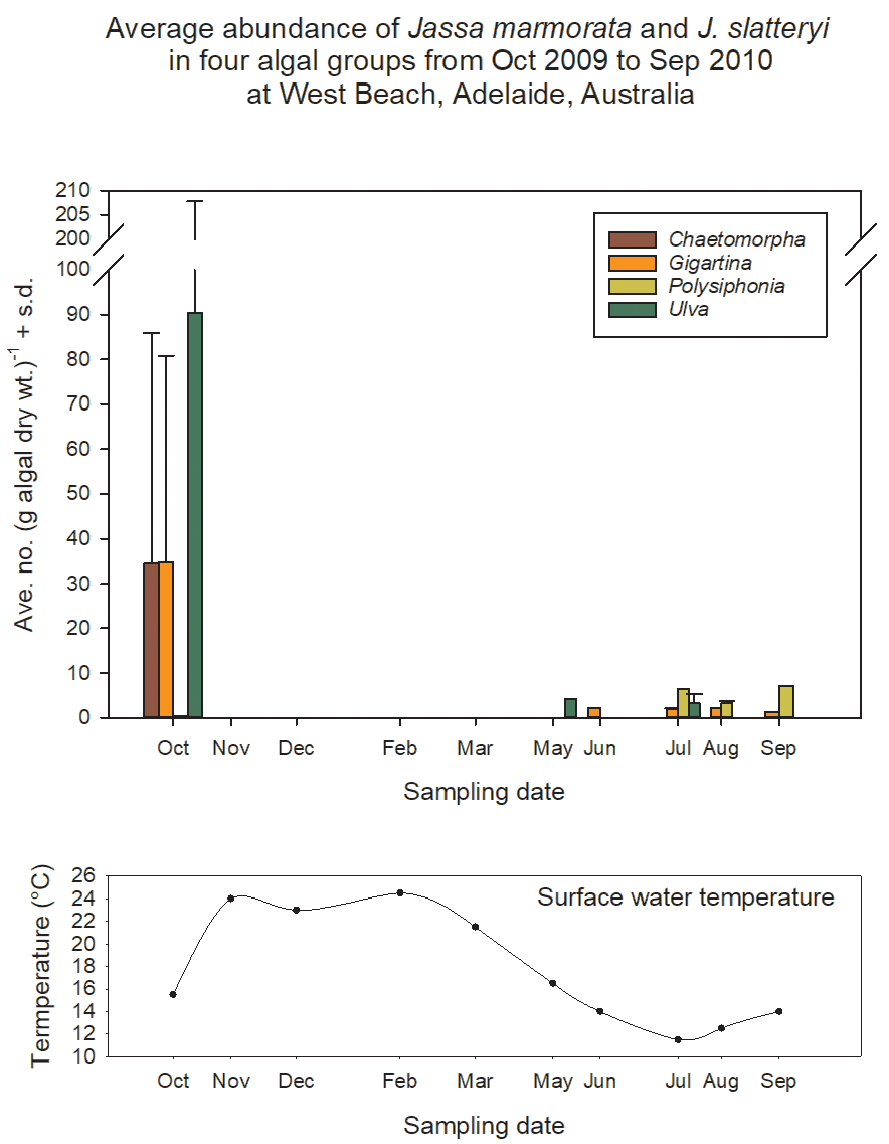
Only two specimens of the ~25,000 specimens of Jassa examined were found to be densely coated in epibionts ( Fig. 21 View FIGURE 21 ). All other specimens appeared to be clean or with much smaller and sparser epibionts. However, specimens collected in Helgoland Harbour ( Germany) often were coated with epibionts. Both of the densely coated specimens were large, major form thumbed males and the epibionts were algal in appearance (green Enteromorpha -like algae or filamentous as in Fig. 21 View FIGURE 21 ). The specimen bearing a green algal coat (found among algae on a floating dock in Adelaide, Australia) was so densely covered that it was barely recognizable. The specimen shown in Fig. 21 View FIGURE 21 was from a fouling community on a natural gas platform in Morecambe Bay, UK In both specimens, the epibionts were concentrated on the anterior of the body, coating the dorsum and second gnathopods. This pattern suggests that the animals were partially tubicolous during the time of infestation. Movement within the tube may have prevented epibiont accumulation on the posterior part of the body. The cleaning action of the first gnathopods may have prevented epibiont growth on body parts within reach, such as the antennae. That epibiont growth was able to establish in such quantity on these males suggests that the males had not molted for some time, and that they were senescent and not able to prevent epibiont settlement.
There are four corrections to Conlan (1990): (1) Sexton and Reid (1951) Plate 12, 5a–5f should be added to p. 2053; (2) specimens from Crooke’s Point, New York (p. 2054) are from Staten Island, New York, not the Hudson River (S. Grabe, pers. comm., date not recorded); (3) J. marmorata is not yet known from Alaska; an Alaskan specimen attributed to J. marmorata in Conlan (1990) and repeated by Fofonoff et al. (2019) is actually the indigenous J. staudei (specimen re-examined May 23, 2018); (4) Conlan (1990) did not recognize that “ J. marmorata ” in the Gulf of Mexico and on the US Atlantic coast from Florida to North Carolina were more likely to be J. valida than J. marmorata , although the two species could overlap in range ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 , 7 View FIGURE 7 , 8 View FIGURE 8 ). Therefore, the identification of specimens from these coasts are suspect. This has been rectified for collections at the Canadian Museum of Nature (CMN) but not elsewhere (see Remarks section for J. valida ). Similarly, “ J. marmorata ” found in the southwestern Gulf of Mexico with J. valida by Winfield et al. (2021) are possibly also J. valida as the specimens they reported were very small (2.36 ± 0.38 mm body length, n = 5) and therefore likely difficult to view the diagnostic terminal seta(e) on the telson that denotes the identification as J. valida (Supplementary Data File S1). It was not possible to borrow the specimens to confirm the identification as the museum collections were closed due to the COVID-19 pandemic.
Aloan that came after Conlan (1990) is a sample of J. marmorata in a collection taken at 13 m depth on a shellmuddy-sand substrate offshore of S„o Paulo, Brazil in 1963. This is the earliest known collection of J. marmorata on the Atlantic coast of South America (Table 3). It is possible that this sample was contaminated by J. marmorata fouling the ship. Ascraping of a small ship in 1985 in this area found J. marmorata on the hull.
Astudy of amphipods inhabiting the moderately polluted Newport Bay in 1954 (now part of Los Angeles) found “ J. falcata ” in small numbers compared to the large populations on pilings in Los Angeles Harbor ( Barnard and Reish 1959). Although not seen, these specimens may be J. marmorata , since it has been found exclusively on other human-modified habitats on the Pacific coast of North America (this study and Fofonoff 2019). Jassa marmorata has been collected in California as far back as 1931 (Table 3).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Tribe |
Ischyrocerini |
|
Genus |