Psilorhynchus sucatio
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00698.x |
|
persistent identifier |
https://treatment.plazi.org/id/03B287ED-FFDD-373E-FCF1-12AA8182AA49 |
|
treatment provided by |
Valdenar |
|
scientific name |
Psilorhynchus sucatio |
| status |
|
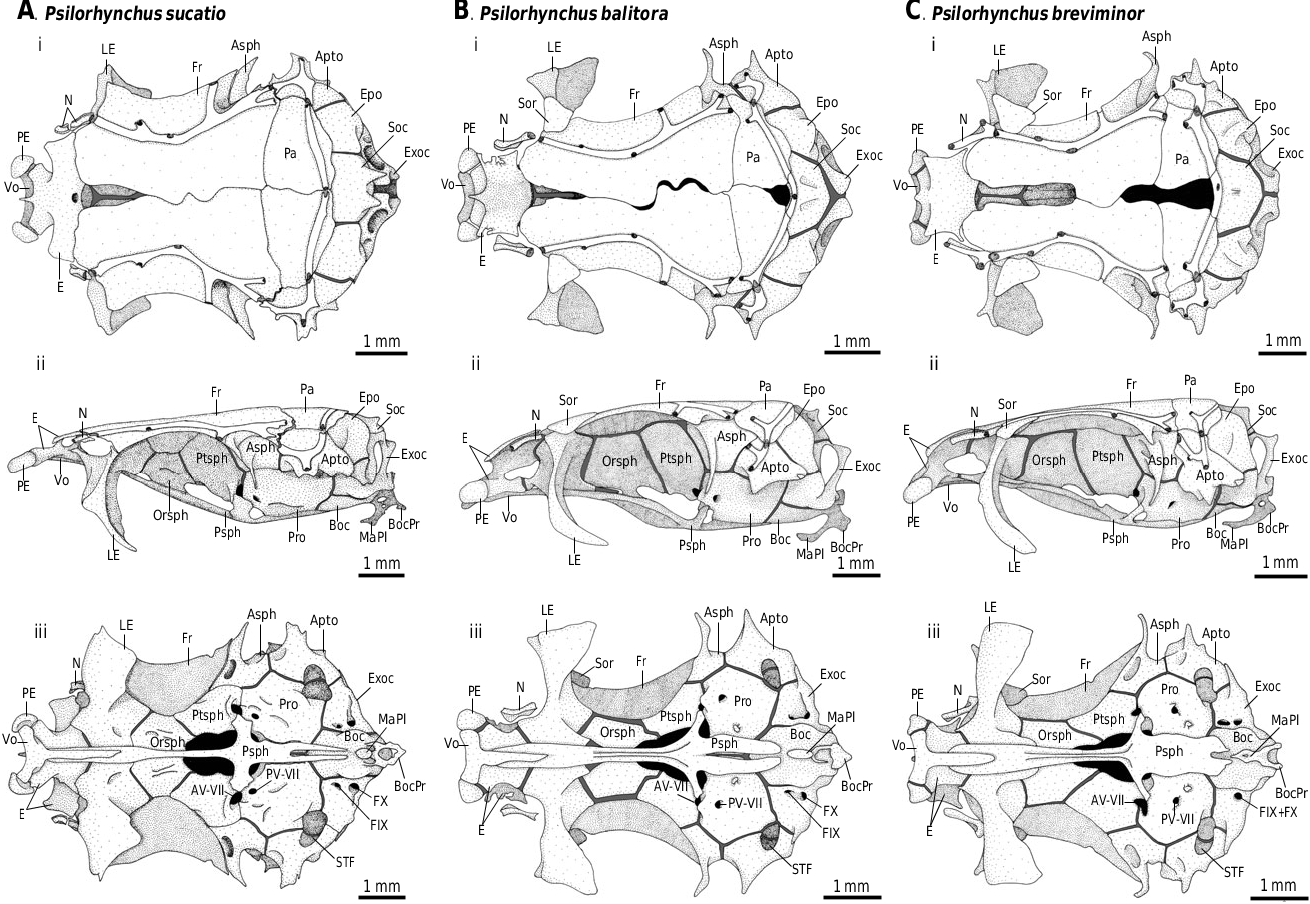
A. Psilorhynchus sucatio View in CoL B. Psilorhynchus balitora
3. Second preethmoid element ( Sawada, 1982: character 52; Siebert, 1987: characters 27, 30): (0) absent; (1) second preethmoid cartilage present; (2) second preethmoid present (CI = 0.67; RI = 0.95).
The majority of cobitoids exhibit a small endoskeletal element anterior to the ethmoid region, situated between the preethmoid and the maxilla, to which it is connected by ligaments ( Sawada, 1982). A similar element is also present in certain cyprinine and gobionine cyprinids ( Ramaswami, 1955a, 1955b; K.W. Conway, pers. observ.). In the majority of taxa examined this element is rod-shaped and round in cross section (Fig. 23D–G). In members of the family Catostomidae the second preethmoid is absent, with only its cartilaginous precursor (the second preethmoid cartilage) being present (Fig. 23B). In all cobitid taxa examined, the element directly anterior to the anterolateral point of the ethmoid complex is cylindrical in shape, and exhibits two posterior articulatory heads (Fig. 23E), one of which articulates with the anterolateral edge of the ethmoid complex, and the other with the anterior head of the autopalatine, which is the usual point of articulation for the preautopalatine in other cobitoid fishes. The ontogeny (and homology) of the second preethmoid element of cypriniform fishes is presently unknown, and it is unclear whether the posteriorly bifurcated element present in cobitids represents the second preethmoid, the preautopalatine, or a compound element resulting from the fusion of the two. For the purposes of this analysis, the posteriorly bifurcated element in question was considered to represent the second preethmoid only, and was coded as such.
4. Articulation between ethmoid complex and frontals ( Sawada, 1982: character 7): (0) absent, posterodorsal edge of ethmoid complex or supraethmoid firmly sutured or tightly abuting anterior edge of frontals; (1) present, posterodorsal edge of ethmoid complex articulating in a shallow facet along anterior edge of frontals (CI = 0.50; RI = 0.90).
In members of the Botiidae , Cobitidae , and certain members of the Nemacheilidae the posterodorsal edge of the ethmoid complex slots into a shallow socket formed by the anterior edge of the frontals, and is reportedly moveable ( Sawada, 1982; Fig. 23D, E). In other cypriniform fishes and in outgroup taxa the posterodorsal edge of the ethmoid complex tightly abuts or is firmly sutured to the anterior edge of the frontals ( Figs 3 View Figure 3 , 23A–C, F, G).
5. Relationship between ethmoid complex and vomer ( Sawada, 1982: character 22): (0) separate; (1) fused (CI = 0.33; RI = 0.84).
In members of the Botiidae , Cobitidae , and certain members of the Nemacheilidae and Balitoridae , there is no separate vomer ( Sawada, 1982; Fig. 25D–G). In other cypriniform fishes and in outgroup taxa the vomer is ventral to the ethmoid complex and the anterior tip of the parasphenoid ( Figs 3 View Figure 3 , 25A–C). Sawada (1982) interpreted the absence of a separate vomer in the botiids, cobitids, and certain nemacheilins as the result of ontogenetic fusion between the vomer and the ethmoid complex. Without ontogenetic information it is unclear whether the vomer has actually fused with the ethmoid complex during the ontogeny of these fishes or is simply absent. Based on the irregular shape of the large median ethmoid element in these fishes, which surrounds the anteriormost tip of the parasphenoid, the hypothesis that the vomer and ethmoid complex are fused is preferred.
6. Contact between orbitosphenoid and ethmoid complex ( Sawada, 1982: character 24): (0) absent; (1) present (CI = 1.00; RI = 1.00).
In members of the Family Balitoridae , Botiidae , Cobitidae , Nemacheilidae and Vaillantella , the lateral ethmoid is displaced laterally and the orbitosphenoid is in direct contact with the ethmoid complex ( Sawada, 1982; Fig. 25D–G). In the remaining cypriniform fishes and in outgroup taxa the orbitosphenoid is separated from the median ethmoid complex by the lateral ethmoid ( Figs 3 View Figure 3 , 25A–C).
7. Anterolateral process of the lateral ethmoid ( Siebert, 1987: character 25; also used by Smith, 1992 in part: character 145): (0) absent; (1) present (CI = 0.12; RI = 0.56).
In the majority of cobitoids the anterolateral edge of the lateral ethmoid extends anteriorly, parallel with the medial face of infraorbital 1 ( Siebert, 1987; Figs 23B, C, F, G, 25B, C, F, G). In other cypriniforms and in all outgroup taxa (except for Elops cf. senegalensis ), the anterior face of the lateral ethmoid is relatively flat ( Figs 3 View Figure 3 , 23A, 25A).
8. Erectile suborbital spine, formed by enlargement of lateral ethmoid ( Sawada, 1982: character 6; Siebert, 1987: character 26): (0) absent; (1) present (CI = 0.50; RI = 0.89).
In members of the Botiidae and Cobitidae the lateral ethmoid is greatly enlarged and mobile. It articulates medially with the anterolateral face of the orbitosphenoid, such that it can be erected anterolaterally ( Sawada, 1982; Fig. 25D, E). In other cypriniform fishes and in outgroup taxa the lateral ethmoid is tightly sutured to the anterolateral edge of the orbitosphenoid, and is immobile ( Figs 3 View Figure 3 , 25A–C, F, G).
9. Preepiphysial fontanelle: (0) absent; (1) present (CI = 1.00; RI = 1.00).
› Figure 23. Dorsal view of upper jaws (left side only) and ethmoid region of selected cypriniform fishes. Anterior to left. A, Balantiocheilos melanopterus, NRM 24037, 36.8-mm standard length (SL). B, Catostomus commersonii, UAIC 14172.01, 62.7-mm SL. C, Gyrinocheilus pennocki, UAIC 14180.51, 73.0-mm SL. D, Yasuhikotakia sidthimunki, UAIC 14166.48, 24.1-mm SL. E, Lepidocephalichthys cf. guntea, UAIC 14180.56, 57.4-mm SL. F, Mesonoemacheilus triangularis, UAIC 14180.57, 49.8-mm SL. G, Homaloptera stephensoni, BMNH 2001.1.15.853–872, 37.9-mm SL. *Meniscuslike structure between palatine process of the maxilla and head of autopalatine. Laminar (unossified) portion of infraorbital 1 shown in light grey in D. Cartilage highlighted in grey. Abbreviations: Apa, autopalatine; E, ethmoid complex; Enpt, endopterygoid; E + Vo, ethmoid complex + vomer Fr, frontal; IO1, infraorbital bone 1; IOC, infraorbital canal ossifications; KE, kinethmoid; LE, lateral ethmoid; MP, mesiopreautopalatine; MPC, mesiopreautopalatine cartilage; Mx, maxilla; N, nasal; PA, preautopalatine; PAC, preautopalatine cartilage; PE, prethmoid; PE2, second prethmoid; PE2C, second prethmoid cartilage; Pmx, premaxilla; SO, supraorbital.
LE Pro Boc 1 mm
In members of the genus Psilorhynchus a fontanelle is present anterior to the preepiphysial bar ( Ramaswami, 1952; Fig. 3 View Figure 3 ). A similarly shaped preepiphysial fontanelle is present in certain characiform, siluriform, and gymnotiform taxa ( Weitzman, 1962; Fink & Fink, 1981), but is absent in other cypriniforms (Fig. 23A–G).
10. Interorbital septum ( Cavender & Coburn, 1992; character 8): (0) interorbital septum formed only by the orbitosphenoid; (1) interorbital septum formed by the orbitosphenoid and a dorsal component of the parasphenoid (CI = 0.33; RI = 0.85).
In members of the cyprinid subfamily Cyprininae , certain catostomids, and certain botiids, the parasphenoid exhibits a large ridge along its dorsal surface that contacts the ventral edge of the orbitosphenoid ( Fig. 24A, D View Figure 24 ). In the remaining cypriniforms and in outgroup taxa the dorsal surface of the parasphenoid is flat and the interorbital septum is formed exclusively by the orbitosphenoid ( Figs 3 View Figure 3 , 24B, C, E–G View Figure 24 ).
11. Ventral keel on parasphenoid: (0) absent; (1) present (CI = 0.12; RI = 0.67).
In certain members of the genus Psilorhynchus ( Fig. 3 View Figure 3 ), all labeonine cyprinids, Cyprinus carpio Linnaeus, 1758 , Rhodeus sericeus (Pallas, 1776) , all members of the Gyrinocheilidae , Yasuhikotakia modesta (Bleeker, 1864) , and Annamia normani (Hora, 1931) , the parasphenoid exhibits a large keel along its ventral surface. The keel originates on the ventral surface of the parasphenoid, slightly posterior to the point of contact between the parasphenoid and the orbitosphenoid, and terminates just anterior to the point of anteriomost contact between the parasphenoid and the prootics. In the remaining cypriniforms and in outgroup taxa the ventral surface of the parasphenoid is relatively flat along its entire length ( Fig. 24 View Figure 24 ).
12. Contact between orbitosphenoid and pterosphenoid ( Sawada, 1982: character 15): (0) present; (1) absent (CI = 1.00; RI = 1.00).
In members of the Cobitidae the orbitosphenoid and pterosphenoid are completely separate ( Sawada, 1982; Fig. 23E). In other cypriniforms and in outgroup taxa the posterior edge of the orbitosphenoid tightly abuts the anterior edge of the pterosphenoid ( Fig. 24A–D, F, G View Figure 24 ).
13. Basisphenoid ( Fink & Fink, 1981: character 7): (0) present; (1) absent (CI = 1.00; RI = 1.00).
In ostariophysan fishes the basisphenoid is absent ( Fink & Fink, 1981). This ossification is present in all non-ostariophysan outgroup members used in this study. 14. Anterior opening of trigeminofacial chamber ( Cavender & Coburn, 1992: character 13): (0) anterior opening of trigeminofacial chamber contained within prootic; (1) anterior opening of trigeminofacial chamber on anterior edge of prootic, its anterior edge bordered by the posterior edge of the pterosphenoid or lateral wing of the parasphenoid (CI = 0.25; RI = 0.40).
In the majority of cypriniform taxa examined the anterior opening of the trigeminofacial chamber is positioned at the anterior edge of the prootic, its anterior edge formed by the posterior edge of the pterosphenoid only ( Figs 3 View Figure 3 , 25A, B, D), or the posterior edge of pterosphenoid plus the lateral wing of the parasphenoid ( Fig. 25E–G). In certain members of the Catostomidae and Gyrinocheilidae , and in all outgroup taxa examined, excluding Denticeps clupeoides , the anterior opening of the trigeminofacial chamber is completely enclosed within the prootic ( Fig. 25C).
15. Postepiphysial fontanelle ( Sawada, 1982: character 10; Smith, 1992: character 66): (0) absent; (1) present (CI = 0.12; RI = 0.65).
In all members of the Botiidae , Catostomidae , and Cobitidae , and in certain members of the Balitoridae , Cyprinidae , Nemacheilidae, and Psilorhynchus , a large cranial fontanelle is present posterior to the epiphysial bar ( Sawada, 1982; Siebert, 1987; Smith, 1992; Kullander, 2008; Fig. 3 View Figure 3 ). This opening is most commonly bordered by the frontal anterolaterally, the parietal laterally, and the supraoccipital posteriorly. In some taxa the postepiphysial fontanelle may be restricted to the posterodorsalmost region of the neurocranium only, without contact to the frontal, or may be bordered posteriorly by the parietal, lacking contact with the supraoccipital ( Kullander, 2008). A postepiphysial fontanelle was present in Distichodus antonii , but was absent in other outgroup taxa included for analysis.
16. Connection between supraorbital sensory canal and otic sensory canal ( Cavender & Coburn, 1992: character 16): (0) present, supraorbital sensory canal connected to otic sensory canal; (1) absent, supraorbital sensory canal disjunct from otic sensory canal (CI = 0.20; RI = 0.56).
In all members of the cyprinid subgrouping Leuciscinae examined, the supraorbital sensory canal is disjunct from the otic sensory canal. In other cypriniform fishes and in outgroup taxa, excluding Hiodon alosoides , the supraorbital sensory canal, when present, is connected with the otic sensory canal, usually via the dermosphenotic ( Gosline, 1974). This character is inapplicable to those taxa in which either the supraorbital sensory canal, the infraorbital sensory canal, and/or the otic sensory canal are absent.
1 mm
·
17. Fenestration of the dilatator fossa ( Stiassny & Getahun, 2007: character 4): (0) absent; (1) present (CI = 1.00; RI = 1.00).
In members of the cyprinid subfamily Cyprininae the frontal exhibits a large fossa close to the point of origin of the dilatator operculi ( Howes, 1987; Figs 24A View Figure 24 , 25A). In labeonine cyprinids the autosphenotic also exhibits a large fossa (termed a ‘double fenestration’ of the dilatator fossa by Stiassny & Getahun, 2007). In other cypriniform fishes and in outgroup taxa the surface of the dilatator fossa is intact ( Figs 3 View Figure 3 , 24B–G View Figure 24 ).
18. Pterotic fossa ( Smith, 1992: character 149): (0) absent; (1) present (CI = 0.50; RI = 0.83).
In members of the Catostomidae and Gyrinocheilidae , a fossa is present on the lateral face of the autopterotic, which serves as the point of origin for the levator operculi (Fig. 23B, C). In other cypriniform fishes and outgroup taxa, excluding the characiform Distichodus antonii , the lateral face of the autopterotic is entire ( Fig. 24A, D–G View Figure 24 ).
19. Supratemporal commissure: (0) located on parietal or extrascapular; (1) located on supraoccipital (CI = 0.33; RI = 0.88).
In numerous cypriniform fishes the point of contact between the supratemporal sensory canals of the left and right sides (the supratemporal commisure) is located on the supraoccipital (e.g. in certain species of Psilorhynchus ; Fig. 3B–F View Figure 3 ). In the majority of outgroup taxa and in certain ingroup taxa (e.g. P. sucatio ; Fig. 3A View Figure 3 ), the supratemporal sensory canal does not extend on to the supraoccipital, and the point of contact between the supratemporal sensory canals of the left and right sides is situated at the suture between the parietals or extrascapulars, anterior to the supraoccipital. The supratemporal commisure is frequently located on the supraoccipital in cypriniform taxa that exhibit a postepiphysial fontanelle, but the two characters do not appear to be correlated (e.g. see Arratia, 1997: fig. 81c). This character is inapplicable to those taxa in which the supratemporal sensory canal is absent.
20. Exoccipital ( Sawada, 1982: character 21): (0) contacting antimere along midline; (1) separate from antimere (CI = 0.5; RI = 0.75).
In members of the Nemacheilidae and Balitoridae , the exoccipital is separated from its antimere along the dorsal midline by the supraoccipital ( Sawada, 1982). In other cypriniform fishes and all outgroup taxa examined, excluding Hiodon alosoides , the exoccipital is in contact with its antimere along the dorsal midline posterior to the supraoccipital ( Fig. 3 View Figure 3 ).
21. Intercalar ( Sawada, 1982: character 23): (0) present; (1) absent (CI = 0.17; RI = 0.78).
In certain members of the Cyprinidae , Cobitidae , Balitoridae, and Nemacheilidae, and in Psilorhynchus , the intercalar is absent ( Figs 3 View Figure 3 , 25E, G). In other cypriniform fishes and in outgroup taxa, excluding Denticeps clupeoides ( Greenwood, 1968) , the intercalar is present ( Fig. 25A–D, H). Contrary to Sawada (1982), the intercalar is present in all members of the Botiidae examined (e.g. Fig. 25D) and in certain members of the Nemacheilidae (e.g. Fig. 25F), and as such its absence does not represent a synapomorphy for the Cobitoidea (sensu Sawada, 1982), as interpreted by Sawada (1982). The single specimen of P. nepalensis examined exhibited an intercalar on the right-side only (see above). Nonetheless, this species was coded as exhibiting the derived condition (1) for this character, in order to avoid polymorphic character coding until additional specimens can be examined.
22. Subtemporal fossa ( Sawada, 1982; character 12; Siebert, 1987: character 41): (0) absent or represented only by a shallow concavity on the ventral surface of the neurocranium; (1) present, represented by a deep concavity on the ventral surface of the neurocranium (CI = 1.00; RI = 1.00).
In Psilorhynchus View in CoL and all members of the Cyprinidae View in CoL a deep subtemporal fossa is present between the posteromedial corner of the prootic, the ventromedial edge of the autopterotic, and the anteroventral edge of the exoccipital ( Ramaswami, 1952; Siebert, 1987; Figs 3 View Figure 3 , 25A, G). In other cypriniform fishes and in out-group taxa, such a fossa is absent or is represented only by a slight depression ( Fig. 25B–F).
23. Basioccipital process: (0) absent; (1) present as long parallel processes, not united to form a canal around the dorsal aorta; (2) present as a large bony structure, forming a canal around the dorsal aorta (CI = 0.40; RI = 0.82).
In members of the Gyrinocheilidae and Botiidae , and in certain members of the Cobitidae and Nemacheilidae , the basioccipital bears a pair of posteriorly directed processes ( Fig. 25C–E). These processes, which remain separate, originate on the posteroventral surface of the basioccipital, and extend posteriorly, parallel with the lateral sides of the dorsal aorta, and terminate slightly posterior to the posteriormost point of the neurocranium. In members of the families Cyprinidae and Catostomidae , and in certain members of the Balitoridae and Nemacheilidae , and in Psilorhynchus , the basioccipital bears a single bony structure on its posteroventral surface, which bears a canal through which the dorsal aorta passes ( Figs 3 View Figure 3 , 25A, B, F). In outgroup taxa and in certain members of the Balitoridae the posteroventral surface of the basioccipital is without such modifications ( Figs 24G View Figure 24 , 25G).
24. Fenestration of the basioccipital process ( Smith, 1992: character 141): (0) absent; (1) present (CI = 1.00; RI = 1.00).
In members of the family Catostomidae the basioccipital process is heavily fenestrated ( Smith, 1992; Figs 24B View Figure 24 , 25B). In other cypriniform fishes the basioccipital process is a solid bony structure, without fenestration ( Figs 3 View Figure 3 , 24A, C–F View Figure 24 , 25A, C–F). This character is inapplicable to outgroup taxa and to members of the Balitoridae that lack the basioccipital process ( Figs 24G View Figure 24 , 25G).
25. Pharyngeal process of the basioccipital process (modified from Stiassny & Getahun, 2007: character 3): (0) absent; (1) present, horizontal in cross section; (2) present, terete in cross section (CI = 0.40; RI = 0.85).
In members of the Cyprinidae and in Psilorhynchus (excluding P. tenura ) the basioccipital process extends far posterior to the point of exit of the dorsal aorta. This posterior portion of the basioccipital process, referred to as the pharyngeal process by Britz & Conway (2009), may be roughly horizontally oriented in cross section, as is the case in the majority of cyprinids and in Psilorhynchus (excluding P. tenura ), or it may be terete in cross section, as in the labeonine cyprinids ( Reid, 1982; Stiassny & Getahun, 2007). In other cypriniform fishes in which the basioccipital process forms a canal around the dorsal aorta the pharyngeal process is absent. This character is inapplicable to outgroup taxa and to cypriniforms in which the basioccipital process is absent.
26. Masticatory plate: (0) absent; (1) present (CI = 1.00; RI = 1.00).
In Psilorhynchus and all members of the Cyprinidae the anteroventral surface of the basioccipital process exhibits a plate-like structure, termed the masticatory plate by Howes (1981). The masticatory plate is quite small in members of the genus Psilorhynchus ( Fig. 3 View Figure 3 ), but is well developed in members of the Cyprinidae ( Fig. 25A). In other cypriniform fishes in which the basioccipital processes form a canal around the dorsal aorta, the masticatory plate is absent ( Fig. 25B, F). This character is inapplicable to outgroup taxa and to cypriniforms in which the basioccipital process is absent.
27. Supraorbital sensory canal: (0) present; (1) absent (CI = 0.33; RI = 0.71).
In certain members of the Catostomidae , Nemacheilidae, and all members of the Cobitidae the supraorbital sensory canal is absent ( Fig. 24B, E View Figure 24 ). In other cypriniform fishes and in outgroup taxa the supraorbital sensory canal is present ( Figs 3 View Figure 3 , 24A, D, F, G View Figure 24 ).
28. Anterolateral process of the frontal: (0) absent; (1) present (CI = 1.00; RI = 1.00).
In members of the Botiidae the frontal exhibits a lateral process, similar in general appearance to the lateral process of the autosphenotic, on its anterolateral edge. This process is quite small in Yasuhikotakia sidthimunki (Klausewitz, 1959) (Figs 23D, 24D), but is very well developed in the other botiid taxa examined. In these later taxa this anterolateral process rims a large percentage of the posterior edge of the lateral ethmoid (e.g. see Sawada, 1982: Figs 9, 10). In other cypriniform fishes and outgroup taxa the anterolateral edge of the frontal is relatively flat, and is without such a process ( Figs 3 View Figure 3 , 23A–C, E–G, 24A–C, E–G).
29. Lateral occipital foramen: (0) absent; (1) present (CI = 0.33; RI = 0.71).
In all cypriniform fishes, excluding members of the Balitoridae and Vaillantella euepiptera (Vaillant, 1902) , the exoccipital bears a large foramen on its lateral face, ventral to the supraoccipital, from which it is separated by a small strut of bone ( Figs 3 View Figure 3 , 24 View Figure 24 ). In all outgroup taxa, excluding the characiform Distichodus antoni , all members of the Balitoridae and V. euepiptera the lateral face of the exoccipital is intact ( Fig. 24G View Figure 24 ).
INFRAORBITAL SERIES
30. Antorbital ( Fink & Fink, 1981: character): (0) present; (1) absent (CI = 0.50; CI = 0.50).
In all cypriniform fishes the antorbital is absent. In outgroup taxa, excluding Hiodon alosoides , the antorbital is present. In certain members of the Nemacheilidae and Balitoridae a dermal ossification is present anterior to the orbit dorsal to the infraorbital sensory canal. This element is interpreted as infraorbital 1, and not the antorbital, based on its close association with the lateral edge of the lateral ethmoid. In members of the Cobitidae , a narrow strip of bone rims the ventrolateral margin of the nasal cavity. Siebert (1987) referred to this ossification as a ‘nasal ribbon’, and hypothesized that this peculiar ossification was a remnant of infraorbital 1, with which I concur.
31. Relationship between anteriormost portion of infraorbital sensory canal and infraorbital 1: (0) anteriormost portion of infraorbital sensory canal completely enclosed on infraorbital 1; (1) anteriormost portion of infraorbital sensory canal completely or partially disjunct from infraorbital 1 (CI = 0.50; RI = 0.93).
In cobitoid fishes, excluding members of the Gyrincheilidae, the anteriormost portion of the infraorbital sensory canal is either partially enclosed on infraorbital 1 (originating anterior to the anteriormost edge of that bone, and/or exiting the bone close to its centre without extending along its posterior half; Fig. 26E, F, H, I View Figure 26 ) or is completely disjunct from infraorbital 1 ( Fig. 26J–L View Figure 26 ). In other cypriniform fishes and in outgroup taxa the anteriormost portion of the infraorbital sensory canal runs along the entire length of infraorbital 1 before continuing on to infraorbital 2 ( Figs 6 View Figure 6 , 26A–D, G View Figure 26 ). This character is inapplicable to taxa in which the infraorbital sensory canal is absent.
32. Nature of infraorbital series posterior to infraorbital 1: (0) infraorbital series posterior to infraorbital 1 represented by four, roughly plate-like bones; (1) infraorbital series posterior to infraorbital 1 represented by a variable number of small tubular ossifications around the infraorbital canal (CI = 0.33; RI = 0.9).
In cobitoid fishes the infraorbital series posterior to infraorbital 1 is represented by a variable number of small bones that are composed almost entirely of sensory-canal ossification ( Siebert, 1987; Fig. 26E, F View Figure 26 ). A similar condition is exhibited by P. sucatio ( Fig. 6A View Figure 6 ) and P. tenura , in which the infraorbital bones posterior to the first are composed soley of sensory-canal ossification. In other cypriniforms and in outgroup taxa the infraorbital bones posterior to infraorbital 1 exhibit plate-like components, in addition to the canal-bearing portion of the bone ( Figs 6B–F View Figure 6 , 26A–D View Figure 26 ). This character is inapplicable to members of the family Cobitidae , excluding Acantopsis choirorhynchus (Bleeker 1854) , and Lefua costata (Kessler, 1876) , which lack infraorbitals posterior to the first.
33. Medial face of infraorbital 2: (0) straight; (1) with shelf, cupping ventral surface of lateral ethmoid (CI = 1.00; RI = 1.00).
In members of the genus Psilorhynchus the medial face of infraorbital 2 exhibits a large shelf that cups the ventral surface of the lateral ethmoid ( Fig. 7), to which it is closely bound with dense connective tissue ( Fig. 4C View Figure 4 ). This medial process is reminiscent of the subocular shelf present on infraorbital 3 in members of the Acanthomorpha ( Smith & Bailey, 1962). In other cypriniform fishes and in out-group taxa the medial face of infraorbital 2 is flat. This character is inapplicable to members of the family Cobitidae , excluding Acantopsis choirorhynchus , and Lefua costata , which lack infraorbitals posterior to the first.
34. Spatial arrangement of Infraorbital 5 and supraorbital ( Chen et al., 1984; Cavender & Coburn, 1992: character 14): (0) in contact or separated only by a short distance; (1) widely separate (CI = 0.33; RI = 0.00).
In the majority of cypriniform fishes the supraorbital (when present) is widely separate from infraorbital 5 ( Figs 6 View Figure 6 , 26A–C, E–G, K View Figure 26 ). In the cyprinids Rasbora cephalotaenia (Bleeker, 1852) and Zacco cf. platypus (Temminck & Schlegel, 1846) the posterior edge of the supraorbital is in contact with the anterior edge of infraorbital 5, or is separated only by a short distance ( Fig. 26D View Figure 26 ). Cavender & Coburn (1992) considered the loss of contact between the supraorbital and infraorbital 5 to represent a synapomorphy of the Cyprinidae , but made note that some rasborin cyprinids exhibited contact between the supraorbital and infraorbital 5 (which they considered to be the plesiomorphic condition). The supraorbital, when present, is disjunct from the posteriormost ossification of the infraorbital series in the majority of cypriniform fishes (it should be noted that in the majority of cobitoid taxa the infraorbital series contains more than five separate ossifications), and as such the validity of this character as a synapmorphy at the level of Cyprinidae is questionable (Conway et al., 2010). This character is inapplicable to taxa in which the supraorbital and/or the infraorbital series are absent.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Psilorhynchus sucatio
| Conway, Kevin W. 2011 |
Cyprinidae
| , Conway & Mayden 2007 |
Psilorhynchus
| McClelland 1839 |